Tiny Genomes May Offer Clues to First Plants and Animals

With just 121 protein-coding genes, the diminutive Tremblaya princeps, a symbiotic bacterium that lives inside specialized cells of the sap-eating mealybug, has the smallest known genome of any cellular organism on the planet. Tremblaya helps to supply the mealybug with essential amino acids and likely receives nutrients and other life-sustaining molecules in return. And even as it tests the lower limits of genome size, the Tremblaya genome may still be shedding genes.
Even more surprisingly, scientists discovered in 2011 that Tremblaya plays host to its own bacterial guest. Called Moranella endobia, the bacterium is smaller in physical size than its host but has more than three times as many genes. Together the three organisms form a complex, co-dependent web; the nested bacteria complement each other and their insect host, creating a genetic patchwork of enzymes needed to produce amino acids lacking in the mealybugs’ sap diet.
Tremblaya presents something of a paradox that some biologists think could help illuminate the evolution of cell parts. The combination of host and symbiont has allowed Tremblaya to cast off many of its genes, surviving with a genome size once thought to be impossible.
“It’s quite remarkable how these bacteria have pushed the lower limit of what we consider to be a viable organism,” said John Archibald, a microbiologist at Dalhousie University in Halifax, Nova Scotia. “Ten years ago, people would have laughed at the idea of bacteria with such a small gene set.”
Given its extreme minuteness and the fact that it must get many essentials from both its host and resident microbes, some suggest that Tremblaya blurs the boundaries between cellular organisms and organelles, specialized structures within cells such as the energy-producing mitochondria. It has been officially designated an endosymbiont, an organism that lives within the cells of another organism. But its genome size resembles that of some organelles. “When do these things stop being bacteria?” asked John McCutcheon, a biologist at the University of Montana in Missoula who studies these organisms.
Indeed, scientists now know that some organelles evolved from endosymbiont bacteria, raising hopes that studying tiny endosymbionts like Tremblaya could shed light on the evolution of those organelles. “There is no bright line between endosymbionts and organelles,” McCutcheon said. “We might be looking at something pretty darn similar to the endosymbiont-to-organelle transition.”
In a paper published today in the journal Cell, McCutcheon and collaborators reveal a striking new level of interdependency among the Tremblaya troika. The mealybug genome appears to include genes from other varieties of bacteria distinct from Tremblaya and Moranella, and the two endosymbiont bacteria may use the protein products of these genes to manufacture nutrients and to make their membranes.
Archibald, who was not involved in the study, described it as “a lot of mixing and matching taking place in evolutionary time.”
Lilliputian Family
Tremblaya is one of a growing family of extremely small endosymbiotic bacteria, discovered within the last seven years, that have challenged scientists’ assumptions about the minimal blueprint of life. “It somehow puts a limit to evolution; how much can you evolve towards efficiency and still be intact?” asked Moselio Schaechter, an emeritus professor of microbiology at Tufts University School of Medicine in Boston, Mass.
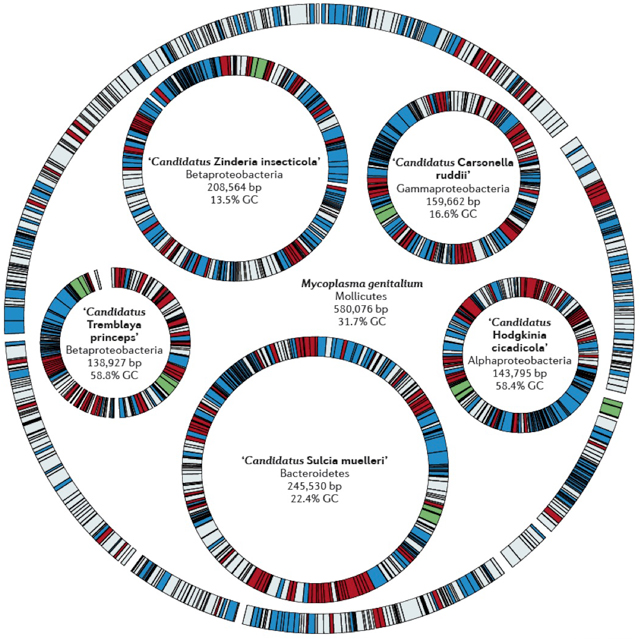
For most of the last 40 years, scientists thought the smallest genomes belonged to bacteria of the Mycoplasma genus. Mycoplasma genitalium, which lives in the human genital tract with just 482 protein-coding genes (compared to about 20,000 in the human genome), became the second bacterial genome ever sequenced, in 1995, and remained the smallest known to scientists for about a decade. “The insect endosymbionts blew the doors off that number,” said McCutcheon. (M. genitalium is still considered to have the smallest genome of a free-living organism — unlike Tremblaya, it can be grown in the lab.)
Many scientists are interested in studying these small-genome organisms for practical reasons. Researchers at the J. Craig Venter Institute, for example, are developing a stripped-down bacterium that can be used as a chassis for biological machines designed to make fuel, medicines or other useful chemicals.
Nature’s most streamlined life forms also provide a lesson in thrift and cooperation. “An endosymbiont like Tremblaya is an illustration of how clever organisms can get,” said Schaechter. “You can see evolution in front of you.”
The collection of bacteria with tiny genomes is surprisingly diverse, having emerged from an array of bacterial ancestries, and having retained and shed a variety of genes. Thanks to the protected environment of the host cell, these organisms tend to evolve rapidly, with the smallest mutating the fastest. Tremblaya and its counterparts have shed many of the genes involved in DNA repair, further accelerating their rates of evolution. They have also lost genes required to make the protective membranes that enclose them and instead are thought to rely on membrane components from the host cell. The genes these organisms retain tend to be involved in producing nutrients for the host, as well as carrying out so-called information repair, which includes DNA replication and the translation of genes into proteins. (Beneficial endosymbionts, such as Tremblaya, are fairly common in invertebrates, but are rare in humans and other vertebrates.)
One of the most intriguing reasons for studying endosymbionts like Tremblaya is to learn about the evolution of mitochondria and chloroplasts, membrane-bound structures within cells that produce energy. Their emergence more than one billion years ago was a foundational event in the development of eukaryotes, which include plants, animals, protists and fungi.
Scientists proposed the idea that these organelles evolved from bacteria as early as the late 1800s, though the theory didn’t become popular until the 1970s. Two key events enabled organelles to develop: The precursor bacteria transferred many of their genes to the host’s genome, and they developed a method of transporting the proteins produced by these and other genes back inside their own membranes. Human mitochondria, for example, have just 13 genes that code for proteins of their own but employ thousands of proteins in their quest to make energy for the cell.
Though their bacterial origins are now well established, many questions remain about the evolution of these organelles. The now ubiquitous mitochondria, for example, evolved just once, and scientists are only able to see the result of the event and not its origin. Tremblaya might illuminate the process that led to mitochondria. “Because it only happened once, it’s hard to know what happened,” said McCutcheon. “Studying endosymbionts gives some insight into that.”
Deep Integration
Tremblaya shares certain attributes with organelles — its genome size is similar to that of some mitochondria and chloroplasts, it is missing a number of vital genes, and its biology is deeply intertwined with that of its host. One obvious difference, however, is that organelles are found in almost every cell in an organism, while endosymbionts, because their main role is to provide nutrients for their hosts, are found only in certain cells. Tremblaya, for example, is found in specialized cells called bacteriomes.
One of the key questions surrounding Tremblaya biology is how the tiny organisms survive. One theory proposes that, like organelles, they saddled their insect hosts with some of their genes, which would help explain their small size and put them on a similar evolutionary path as mitochondria. McCutcheon’s team saw no evidence for this in their Cell paper, but what they found is even more complicated.
Alluring Amoeba
Tremblaya isn’t the only example of potential organelle evolution in progress. Nearly 40 years ago, scientists discovered that an amoeba called Paulinella chromatophora plays host to a photosynthetic structure that in some ways resembles cyanobacteria, the original ancestors of chloroplasts. Genetic studies conducted over the last 10 years have shown that this structure did indeed originate from cyanobacteria, but much more recently than traditional chloroplasts, and that the ancestral bacteria appear to have transferred some genes to their amoeba host. (Their genomes, which house around 850 genes, remain larger than those of most organelles, however.)
A paper published last year in the Proceedings of the National Academy of Sciences by Eva Nowack and Arthur Grossman of the Carnegie Institution for Science in Stanford, Calif., suggests that these organisms also satisfy the second requirement for organelles — they import proteins from host genes. The findings challenge the conventional notion that photosynthetic organelles developed only once in the course of evolutionary history.
It’s still not clear exactly how Paulinella imports these proteins. “It’s very difficult to recognize novel protein-import machinery,” said Archibald. Because Paulinella’s endosymbionts evolved from different ancestors than traditional chloroplasts, “you can’t just look for a parts list based on chloroplasts.”
The mealybug genome harbors 22 genes from bacteria with ancestors unrelated to Tremblaya and Moranella, but these genes, which code for proteins involved in the production of essential nutrients and the synthesis of the cellular wall enclosing the bacteria, “fit with things missing in the symbionts,” said McCutcheon. “These [organisms] are not getting small by transferring genes to the host,” he said. “They are getting small by coopting bacterial genes in the host, a level of complexity that we would not have predicted.”
The findings provide a more detailed understanding of the symbionts’ differences and similarities to organelles. Tremblaya hasn’t transferred genes to its host, a defining property of organelles. But, like mitochondria, McCutcheon’s findings suggest, Tremblaya coopts some host-derived proteins that originally came from other types of bacteria. “This is a murky gray area; the host encodes genes the symbiont needs to survive, which suggests the hosts target proteins to the organism,” said Patrick Keeling, a biologist at the University of British Columbia, who was not involved in the Cell study. “That’s something organelles do but not usually endosymbionts.”
Not everyone agrees that understanding Tremblaya may help illuminate the evolution of organelles. William Martin, a biologist at the University of Düsseldorf in Germany, wrote in an email that Tremblaya is instead “a beautiful contrast to organelles.” He noted, for example, that organelles import the vast majority of proteins from the host. Tremblaya also seems to import some proteins, but “it’s a far cry from the protein import apparatus of chloroplasts and mitochondria,” he wrote.
Even if Tremblaya doesn’t meet all the requirements of an organelle, the bacterium does seem to be integrated with its host in a way that is similar to organelles.
“They appear to be more integrated at more levels than any other endosymbiont and they share that characteristic with organelles,” said Keeling, who added that the question of what to call Tremblaya and other tiny intracellular dwellers can “get some people’s blood boiling.” (He said he personally doesn’t care what Tremblaya is called.)
Though at some level it’s a question of semantics, the debate also touches on the deeper question of what it means to be alive.
The intracellular parasites that Keeling studies, which also have reduced genomes and are unable to produce their own source of energy or survive without their hosts, are typically thought of as organisms. “But no one refers to mitochondria as an organism because it’s so integrated with its host,” he said.
The difference is that Keeling’s parasites steal energy, in the form of a molecule called adenosine triphosphate or ATP, from their hosts, but they possess the necessary genes to replicate DNA. An organelle, on the other hand, relies on proteins provided by the host to replicate its DNA. “We have arbitrarily decided that stealing ATP from a host constitutes an organism and stealing proteins does not,” said Keeling. “It’s truly just a matter of degrees.”
This article was reprinted on ScientificAmerican.com.