Scientists Reveal Structure of Pain Sensor

The fiery sting of a habanero pepper, the scalding heat of a boiling teapot, the excruciating bite of the earth tiger tarantula, and even the heightened sensitivity to touch following a sunburn — all of these painful sensations are made possible by a sophisticated molecular machine operating in nerve fibers in the skin and tongue.
Known as TRPV1, the protein was discovered more than 15 years ago. Although scientists knew that it could sense heat and various chemicals, exactly how it worked remained a mystery.
In December, however, scientists reported creating a high-resolution image of the protein’s structure for the first time. Like the blueprint of a motor, that information should help researchers understand how the tiny apparatus can respond to such a wide array of signals — from temperature to toxins — and the role it plays in both acute and chronic pain. The results could ultimately lead to new painkillers, potentially without the troublesome side effects of opiates.
David Julius began hunting for TRPV1 close to 20 years ago. At the time, scientists had for decades been using capsaicin, the molecule that gives chili peppers their heat, to study pain. But little was known about how it triggered that sensation. Other scientists had already tried and failed to find the molecule that binds to capsaicin, known as its receptor, but that only enticed Julius to take on the challenge. “People had looked for it for many years, and it took on a mythical glow,” said Julius, a biologist at the University of California, San Francisco. “What is this elusive thing?”
He and his team reported hitting the jackpot in 1997, identifying a member of a family of receptors known as TRP (transient receptor potential) ion channels, which had been little studied in mammals. “They were kind of enigmatic,” said Julius, whose office at UCSF is scattered with capsaicin-themed gifts like chili pepper neckties. His lab has since pioneered the study of TRPV1 and some of its cousins, which can detect cold as well as natural products such as menthol, garlic and wasabi.
A Guarded Passage
Mammals have nearly 30 different TRP channels scattered throughout different parts of the body. Six to nine are involved in sensing temperature. TRPV1 is by far the best studied; scientists are learning more about the other TRP channels, but the function of many remains unknown.
Wasabi, Horseradish and Mustard
TRPV1 isn’t the only temperature-sensitive TRP channel. Five years after isolating TRPV1, Julius and Patapoutian independently discovered that its molecular cousin, known as TRPM8, senses both cold and menthol, a cooling compound derived from mint. (Like capsaicin, menthol is used in over-the-counter pain salves.) “That solidified the idea that thermosensation is the province to a large degree of TRP ion channels,” Julius said.
Another member of the family, TRPA1, senses wasabi, horseradish and mustard, and in some animals, temperature. Some evidence suggests that it even helps snakes sense infrared light. Researchers have since confirmed the different TRP channels’ role in temperature sensing by wiping out these receptors in mice, creating animals that are less sensitive to heat or cold.
In humans, mutations in different TRP channels are linked to a variety of disorders, including problems with the skin, kidneys and skeleton. “In some ways, we know more about what happens with mutation than we know about the real role for these channels,” Julius said.
The TRPV1 molecule, found in the nerve fibers that suffuse the skin and tongue, forms a channel that acts like a gated passage between the inside and outside of the neuron. When you bite into a chili pepper, capsaicin binds to the channel and opens the gate. Charged particles rush into the cell, triggering electrical activity that sends messages of pain to the brain. The same thing happens when you sip a cup of scalding tea, with heat itself opening the gate.
But TRPV1 doesn’t simply sense chemicals or temperature. It acts like a tiny computer, collecting information about the environment to help protect us from further injury. It can make certain sensations feel more painful, warning us to take care. Scientists know from previous experiments that the channel can act like a volume knob to amplify pain; dousing it with capsaicin, for example, lowers its threshold for heat. That’s why hot tea feels even hotter after eating a chili pepper. Damage to the skin, such as sunburn, has a similar effect. It releases inflammatory molecules that act like capsaicin, making the channel easier to open and the skin hypersensitive to additional dangers, like heat or chemicals.
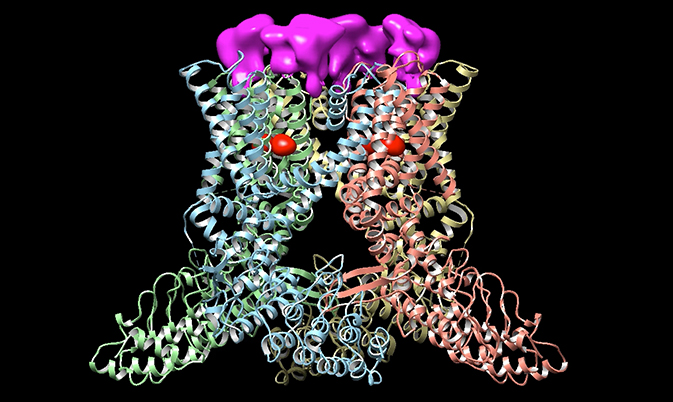
The newly resolved structure helps to explain how the channel changes shape in response to different chemicals, revealing a sophisticated system for how different triggers open the gate. Rather than a simple entrance, the TRPV1 channel is guarded by two sets of doors, similar to a double airlock, according to the new findings, published in Nature in December. The channel has two gates — one faces the inside of the cell and one faces the outside. Both have to open for ions to flow through.
Some chemical triggers, such as capsaicin or the inflammatory molecules that the immune system releases after an injury, seem to act like WD-40, encouraging the gates to swing open more frequently. Others, such as spider toxins, act more like a doorstop to keep them open. In one of the new studies, researchers captured images of TRPV1 in action using three different triggers: capsaicin, a capsaicin-like molecule from succulents, and a spider toxin. They found that capsaicin and the similar molecule both bound near the inner gate, while the spider toxin bound near the outer gate. Exposure to these chemicals increases the likelihood that both gates will be open, which makes it more sensitive to heat or other chemicals.
“It’s an amazing technical feat,” said Ardem Patapoutian, a neuroscientist at the Scripps Research Institute in San Diego who was not involved in the studies. “It’s a major finding for anyone working on the structure of membrane proteins.”
One of TRPV1’s most unusual properties is its ability to sense heat — it is one of only a handful of molecular channels so well tuned to temperature. Although it seems obvious in hindsight, before Julius’ team discovered the capsaicin receptor, no one had anticipated that the same molecule would respond to chili peppers and high temperatures. “Most receptors we know of are activated by chemicals like small molecules and proteins,” said Patapoutian, who is also affiliated with the Howard Hughes Medical Institute. “Here, we have molecules that are exquisite temperature sensors — they act as the body’s thermometers.”
Scientists are now trying to figure out how heat changes the shape of the channel — they already know hot temperatures can open it, but they don’t know exactly how. They also want to examine how molecules produced by our body in response to injury affect the sophisticated sensor and, in turn, our perception of pain.
Structural Success
The Julius lab sports an eclectic mix of chemistry diagrams and photos of animals that his students have studied, such as snakes and vampire bats. Those animals reflect one of the methods that the researchers have used to figure out how the channel works. Comparing the DNA sequence of capsaicin receptors from various animals can pinpoint some of the most important parts of the channel. Birds, for example, can’t detect the chemical, so analyzing differences in the bird and human sequences can help identify the parts that are crucial for sensing the spicy compound. Introducing genetic errors that alter the protein’s ability to bind capsaicin or other chemicals also highlights regions that are essential for different functions. But this approach doesn’t reveal what the channel looks like or how it changes when bound to capsaicin — such a picture had proved elusive.
About six years ago, Erhu Cao, one of Julius’ postdoctoral researchers, set out to decipher the channel’s structure. Cao first tried the most common technique for studying the architecture of complex proteins, called X-ray crystallography. However, that failed. Julius speculates that the same property that gives the channel its power — its ability to change shape in response to different triggers — thwarted efforts to capture a clear picture of it. Fortunately, only two floors above the Julius lab, the biophysicist Yifan Cheng had been refining a newer technique called single-particle electron cryomicroscopy. Cheng’s recent advances in imaging technology achieved the resolution needed to capture a membrane protein in atomic detail. “Seeing the initial [images] with and without the toxin bound to it was breathtakingly beautiful,” Julius said. “It gives us a lot of information about the structurally important parts of the channel, like which parts move as it goes through transition.”
For most membrane channels, scientists have been limited to studying the structure in one conformation — open or closed. But using the new technique, the researchers captured three states: open, closed and partly open. “We can get the first view of where the compounds from chili peppers bind,” said Rachelle Gaudet, a structural biologist at Harvard University who was not involved in the studies.
With this technique, scientists can now explore other TRP channels and how variations in shape influence what they do. “Each TRP channel has a large portion inside the cell, and those vary a lot between different types of TRP channels,” Gaudet said. “Probably a lot of diversity in function comes from those intracellular portions.”
It should also be possible to study the architecture of many other molecular machines at the atomic level. “I think it will open a tremendous opportunity to study other membrane proteins,” Cheng said.
When Pain Goes Astray
Capsaicin seems to skirt the line between pain, pleasure and relief. The compound is found in an array of playfully named hot sauces — “congealed dragon’s blood” ranks third on ChilliWorld’s top-10 list of hottest hot sauces — as well as in over-the-counter pain ointments. Julius theorizes that after triggering an initial burning sensation, capsaicin can have the longer-term effect of desensitizing the TRPV1 channel, as well as the nerve fiber at large, silencing these pain sensing nerves.
Developing pain medication aimed at controlling TRPV1 and other TRP receptors, which lie in our peripheral nervous system, could provide a better alternative to opiates, pain medications that are effective but influence overall nerve activity and can affect breathing, alertness and other essential functions. “The closer you are to the periphery, the better chance you have to intercede in a pain-specific way,” without dangerous side effects, Julius said.
However, the compounds could carry their own drawbacks. Some early candidates have shown problematic side effects during human testing; some people taking the drugs developed abnormally high temperatures or couldn’t properly detect dangerous heat, such as scalding water. The newly resolved structure should help drug makers find chemicals that block the inflammatory signals that sensitize the channel but have no effect on its heat sensors. “Once you understand the structure, you can think about doing more structure-based drug design,” Julius said. “Pain has taken a leap into the molecular era.”
This article was reprinted on Wired.com.