Did Neurons Evolve Twice?
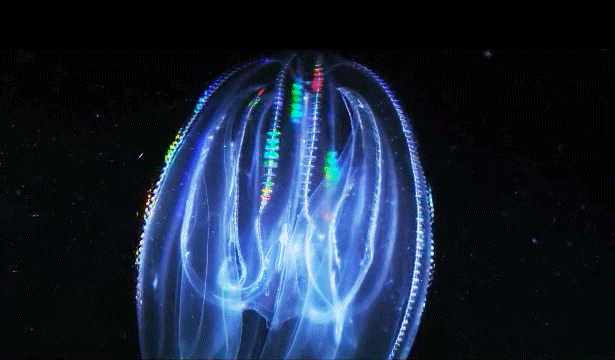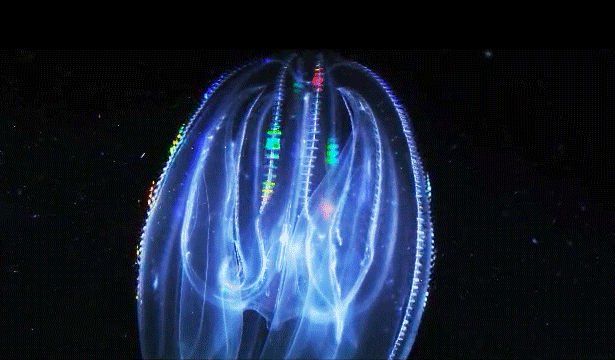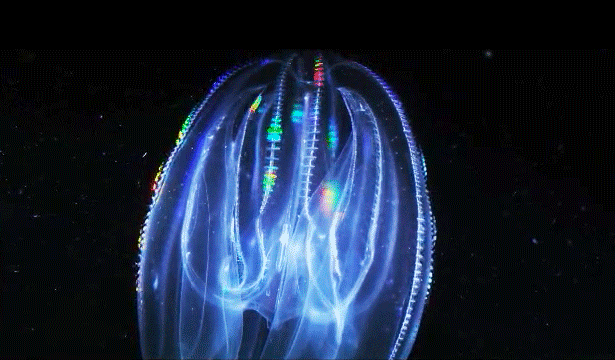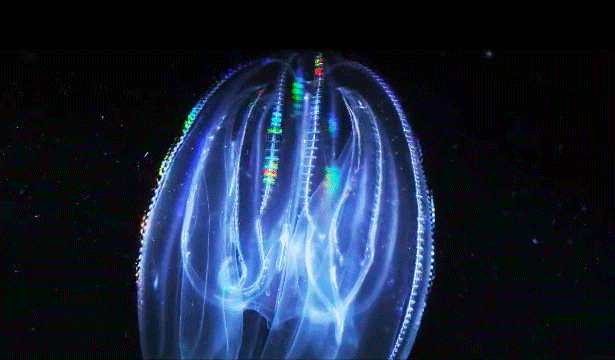
When Leonid Moroz, a neuroscientist at the Whitney Laboratory for Marine Bioscience in St. Augustine, Fla., first began studying comb jellies, he was puzzled. He knew the primitive sea creatures had nerve cells — responsible, among other things, for orchestrating the darting of their tentacles and the beat of their iridescent cilia. But those neurons appeared to be invisible. The dyes that scientists typically use to stain and study those cells simply didn’t work. The comb jellies’ neural anatomy was like nothing else he had ever encountered.
After years of study, he thinks he knows why. According to traditional evolutionary biology, neurons evolved just once, hundreds of millions of years ago, likely after sea sponges branched off the evolutionary tree. But Moroz thinks it happened twice — once in ancestors of comb jellies, which split off at around the same time as sea sponges, and once in the animals that gave rise to jellyfish and all subsequent animals, including us. He cites as evidence the fact that comb jellies have a relatively alien neural system, employing different chemicals and architecture from our own. “When we look at the genome and other information, we see not only different grammar but a different alphabet,” Moroz said.
When Moroz proposed his theory, evolutionary biologists were skeptical. Neurons are the most complex cell type in existence, critics argued, capable of capturing information, making computations and executing decisions. Because they are so complicated, they are unlikely to have evolved twice.
But new support for Moroz’s idea comes from recent genetic work suggesting that comb jellies are ancient — the first group to branch off the animal family tree. If true, that would bolster the chance that they evolved neurons on their own.
The debate has generated intense interest among evolutionary biologists. Moroz’s work does not only call into question the origins of the brain and the evolutionary history of animals. It also challenges the deeply entrenched idea that evolution progresses steadily forward, building up complexity over time.
The First Split
Somewhere in the neighborhood of 540 million years ago, the ocean was poised for an explosion of animal life. The common ancestor of all animals roamed the seas, ready to diversify into the rich panoply of fauna we see today.
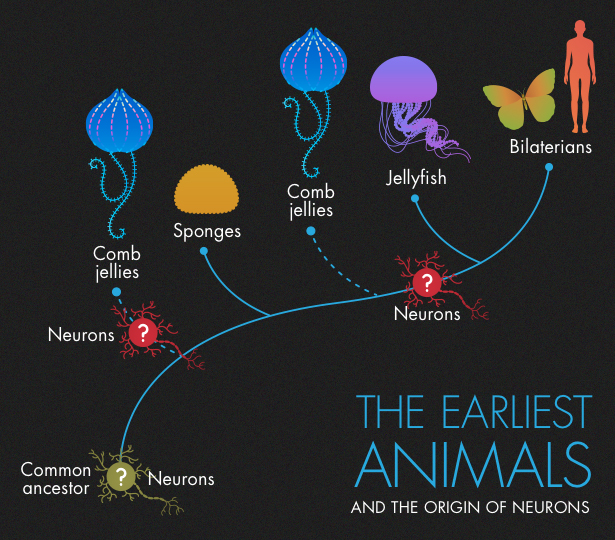
Scientists have long assumed that sponges were the first to branch off the main trunk of the animal family tree. They’re one of the simplest classes of animals, lacking specialized structures, such as nerves or a digestive system. Most rely on the ambient flow of water to collect food and remove waste.
Later, as is generally believed, the rest of the animal lineage split into comb jellies, also known as ctenophores (pronounced TEN-oh-fours); cnidarians (jellyfish, corals and anemones); very simple multicellular animals called placozoa; and eventually bilaterians, the branch that led to insects, humans and everything in between.
But sorting out the exact order in which the early animal branches split has been a notoriously thorny problem. We have little sense of what animals looked like so many millions of years ago because their soft bodies left little tangible evidence in rocks. “The fossil record is spotty,” said Linda Holland, an evolutionary biologist at the Scripps Institution of Oceanography at the University of California, San Diego.
To make up for our inability to see into the past, scientists use the morphology (structure) and genetics of living animals to try to reconstruct the relationships of ancient ones. But in the case of comb jellies, the study of living animals presents serious challenges.
Little is known about comb jellies’ basic biology. The animals are incredibly fragile, often falling to pieces once they’re caught in a net. And it’s difficult to raise them in captivity, making it nearly impossible to do the routine experiments that scientists might perform on other animals.
For a long time comb jellies were thought to be closely related to jellyfish. With their symmetrical body plans and gelatinous makeup, the two species outwardly resemble one another. Yet the animals swim and hunt differently — jellyfish have stinging tentacles, while comb jellies have sticky ones. And at the genome level, comb jellies are closer to sponges, which have no nervous system at all.
In comb jellies or in any other animal, an evolutionary analysis that relies on morphology might lead to one evolutionary tree, while one that uses genomic data, or even different kinds of genomic data, might lead to another. The discrepancies often spark heated debate in the field.
One such debate emerged in 2008, when Mark Martindale, now director of the Whitney Laboratory, Gonzalo Giribet, an evolutionary biologist at Harvard University, and collaborators published a study that analyzed gene sequences from 29 different animals. After considering the genetic data, the researchers proposed a number of changes to the animal tree.
By far the most controversial of these changes was the suggestion that ctenophores should replace sponges as the earliest branch of animals. If evolution increases complexity over time, as biologists have traditionally believed, then an apparently simple organism like the sponge should predate a seemingly more complex organism like the comb jelly. Martindale and Giribet’s genetic data suggested otherwise, but critics were dubious. “We were pretty much ridiculed by the entire scientific community,” Martindale said.
Martindale and his collaborators needed to gather more evidence for their proposal. They convinced the National Institutes of Health to sequence the genome of a comb jelly, the sea walnut, which was published in Science in 2013. Moroz and his collaborators published a second ctenophore genome, the sea gooseberry, in Nature in 2014. Both papers, which employed more extensive data and more sophisticated analysis methods than the 2008 effort, support the ctenophore-first tree. A third paper analyzing publicly available genome data and posted to the preprint server biorxiv.org earlier this year also supports the idea that comb jellies branched off first.
In light of the new evidence, scientists are beginning to take the idea seriously, although many in the field say there isn’t enough data to make any strong claims. This viewpoint has been reflected in a flurry of review articles published over the last year, many of which contend that comb jellies aren’t really the oldest branch; they just appear to be.
Comb jellies have evolved more rapidly than the other ancient animal groups, meaning that their gene sequences changed quickly over time. This in turn means that the genetic analysis of their place in the evolutionary tree could be subject to a computational artifact called “long-branch attraction,” a sort of glitch that can pull rapidly evolving organisms to the base of the tree. “Long-branched animal groups are often difficult to place,” said Detlev Arendt, an evolutionary biologist at the European Molecular Biology Laboratory in Germany. “So far, the phylogenetic data is not really conclusive on where [comb jellies] belong.”
Scientists hope that more data — including genomes of additional ctenophore species — will help resolve the deepest branches of the animal tree. And that, in turn, could have profound implications for our understanding of neurons and where they came from. “The branching order has a major influence on how we interpret the evolution of the nervous system,” said Gáspár Jékely, a biologist at the Max Planck Institute for Developmental Biology in Germany.
Indeed, even those who agree that comb jellies came first disagree on the question of how neurons arose.
The Spark of Thought
The creation of neurons was a remarkable event in animal evolution. These cells can communicate — receiving, transmitting and processing information using a precise chemical and electrical language. Their power derives from the complex network they create. “A single neuron is like the sound of one hand clapping,” Martindale said. “The whole idea is that you put a bunch of them together and they can do things that a few single cells cannot.”
This level of complexity requires an unlikely confluence of evolutionary events. Mechanisms must arise that not only physically connect cells, but allow them to transmit and interpret signals. “The reason most people do not think that they could have evolved multiple times is the idea that neurons talk — specifically to other neurons,” Martindale said.
That’s what makes Moroz’s proposal — that neurons evolved twice, once in comb jellies and once in other animals — so controversial.
According to Moroz’s version of the evolutionary tree, animals started off with a common ancestor that had no neurons. Comb jellies then split off and went on to develop their strange brand of neurons. After that, ancestors of sponges and placozoans branched off. Like their ancestors, they lacked neurons. Rudimentary neurons, or protoneurons, then evolved for a second time in the ancestors of jellyfish and bilatarians, forming the basis of the nervous system found in all subsequent descendants, including humans. “In my opinion, it’s simpler and more realistic that the common ancestor had no nervous system,” Moroz said. (He thinks that even if comb jellies split off after sponges, they still evolved neurons independently.)
But some scientists who believe that ctenophores branched off first paint a different picture. They suggest that the common ancestor to all animals had a simple nervous system, which sponges subsequently lost. Comb jellies and the remaining branch, which includes our ancestors, the bilaterians, built on those protoneurons in different ways, developing increasingly sophisticated nervous systems.
“The ctenophores-first idea, if correct, suggests something really interesting going on,” said Christopher Lowe, a biologist at the Hopkins Marine Station at Stanford University. “Both interpretations are profound.” On the one hand, two independent origins of neurons would be surprising because it seems unlikely that the precise sequence of genetic accidents that created neurons could happen more than once. But it also seems unlikely that sponges would lose something as valuable as a neuron. “The only example we know from bilaterians where the nervous system was lost completely is in parasites,” Lowe said.
The two possibilities reflect a classic conundrum for evolutionary biologists. “Did this animal lose something or not have it to begin with?” Holland said. In this particular case, “I find it’s hard to take a stand,” she said.
Evolution is rife with examples of both loss and parallel evolution. Some worms and other animals have shed regulatory molecules or developmental genes employed by the rest of the animal kingdom. “It’s not unprecedented for important complements of genes to be lost in major animal lineages,” Lowe said. Convergent evolution, in which natural selection produces two similar structures independently, is fairly common in nature. The retina, for example, evolved independently several times. “Different animals sometimes use extremely different toolkits to make morphologically similar neurons, circuits and brains,” Moroz said. “Everyone accepts the eye case, but they think the brain or neuron only happened once.”
Moroz’s primary evidence for an independent origin of neurons in comb jellies comes from their unusual nervous systems. “The ctenophore’s nervous system is dramatically different from any other nervous system,” said Andrea Kohn, a molecular biologist who works with Moroz. Comb jellies appear to lack the commonly used chemical messengers that other animals have, such as serotonin, dopamine and acetylcholine. (They do use glutamate, a simple molecule that plays a major role in neuronal signaling in animals.) Instead, they have genes that are predicted to produce a slew of neural peptides, small proteins that can also act as chemical messengers. “No other animal except in this phylum has anything like that,” Kohn said.
But critics question this assertion as well. Perhaps comb jellies really do have the genes for serotonin and other neural signaling molecules, but those genes have evolved beyond recognition, Arendt said. “It could just mean [that comb jellies] are highly specialized,” he said.
Scientists on all sides of the debate say that it can only be answered with more data, and, more importantly, a better understanding of comb jelly biology. Even though they share some genes with model organisms, such as mice and fruit flies, it’s unclear what those genes do in comb jellies. Nor do scientists understand their basic cell biology, like how comb jelly neurons communicate.
But the ongoing debate has sparked interest in ctenophores, and more researchers are studying their nervous systems, development and genes. “Moroz and collaborators have shined the light on this part of the tree, which is a good thing,” Holland said. “We shouldn’t ignore those guys down there.”
Correction on March 26, 2015: An original caption describing two comb jellies reversed their positions. The sea gooseberry is on the left, the lobed comb jelly on the right.
This article was reprinted on BusinessInsider.com.