‘Fullertubes’ Join the Family of Carbon Crystals
The newly identified carbon crystals fulfill the geometric rule that 12 pentagons and an even number of hexagons can form a closed shell.
Ruth Yaro for Quanta Magazine
Introduction
Carbon can arrange itself into one of the hardest materials in nature, or into one so soft that children inscribe trails of it on paper. Several decades ago, scientists started wondering: Aside from diamond and graphite, what other crystalline forms might carbon take?
In 1985, they had their first answer. A group of chemists discovered little hollow spheres constructed of 60 carbon atoms that they dubbed buckminsterfullerenes, or buckyballs or fullerenes for short. (The crystals resembled geodesic domes, popularized by the architect R. Buckminster Fuller.) A new field of chemistry sprang up around the nanometer-wide spheres, as researchers raced to discover properties and applications of what’s been called the most beautiful molecule.
Bigger fullerenes were found. Then, a few years later, a paper by the Japanese physicist Sumio Iijima sparked interest in a related carbon form, initially dubbed buckytubes but now known as carbon nanotubes: hollow cylinders made of a honeycomb lattice of carbon atoms that’s rolled up like a toilet paper tube.
The carbon crystals had a spectrum of electrical, chemical and physical properties that no other element seemed to match. Excitement around carbon nanoscience was cranked still higher when three of the discoverers of buckyballs, Robert Curl, Harold Kroto and Richard Smalley, received the 1996 Nobel Prize in Chemistry. Then in 2004, the physicists Andre Geim and Konstantin Novoselov found a way to isolate flat sheets of carbon atoms — a crystal known as graphene — igniting another explosion of research that has sustained itself ever since, and earning themselves the 2010 physics Nobel.
Recently, chemists discovered yet another type of carbon crystal — this time, to much less fanfare. Most of the carbon experts contacted for this story still hadn’t heard of it. And so far, the entire global supply probably amounts to milligrams, about the mass of a handful of house flies.

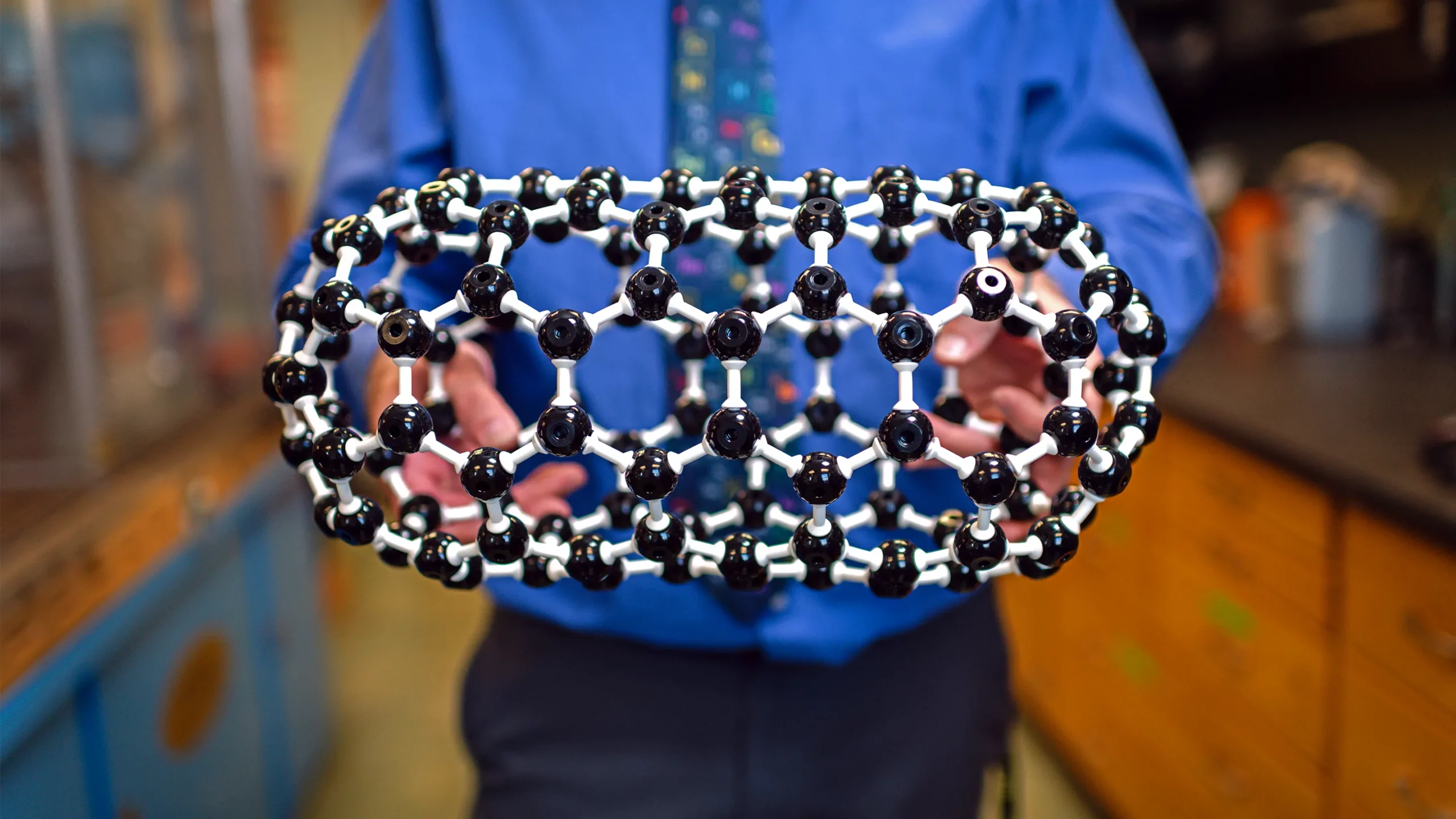
Steven Stevenson, a chemist at Purdue University, proudly holds a model of a fullertube, a kind of carbon crystal he discovered in 2020.
Ruth Yaro for Quanta Magazine
These newest carbon structures fall somewhere between spherical fullerenes and cylindrical nanotubes; they’re “a nanoscale marriage” of the two that’s shaped like a medicine capsule, according to Harry Dorn, a chemist at the Virginia Polytechnic Institute and State University who collaborates with Steven Stevenson of Purdue University, the initial discoverer of the molecules. Stevenson and Dorn have named the crystals fullertubes.
Fullertubes combine the best features of fullerenes and nanotubes. Or the worst of both. Or maybe a bit of the good and bad from each — it depends on who you ask. How or whether their properties will be useful remains to be seen. It’s a place we’ve been before, and arguably still are, with fullertubes’ celebrated carbon relatives.
Mining for Fullertubes
The center of the fullertube world is a chem lab the size of a living room at Purdue’s Fort Wayne, Indiana, campus. There, Stevenson and his small cadre of undergraduates collect and taxonomize the newfound molecules, which consist of hemispherical caps on the ends of cylinders of various widths and lengths.
In 2020, Stevenson and collaborators announced the first member of the fullertube family, a 90-atom molecule that’s essentially two halves of a buckyball connected by a 30-atom nanotube midsection. They found the molecule along with two larger siblings made of 96 and 100 carbon atoms, respectively.
This year, Stevenson and Dorn described two more fullertubes, both consisting of 120 carbon atoms. Their studies show that the narrower of these pill-shaped molecules is electrically conductive, while the wider, shorter one is — intriguingly — a semiconductor, meaning it could potentially be used for transistors and other electronic devices. Fullertubes also have a range of optical and tensile properties that the researchers are still exploring.
Merrill Sherman/Quanta Magazine
Merrill Sherman/Quanta Magazine
James Heath of the Institute for Systems Biology in Seattle, who helped isolate the first fullerenes as a graduate student working with Curl and Smalley in 1985, called the new fullertubes “lovely structures” that follow the same geometric rule that led him and his colleagues to search for fullerenes in the first place: the rule that 12 pentagons and an even number of hexagons can form a closed shell. (Buckyballs, for example, have the same pattern of hexagons and pentagons as a soccer ball. Fullertubes maintain the rule while adding extra belts of hexagons.)
The molecules have been under chemists’ noses for years, hiding in the same special carbon soot that’s long been the primary source of fullerenes. But in 2020, Stevenson finally figured out how to pick out the tubular capsules from among the much more abundant fullerenes. The “magical” process, as he calls it, is to “react away anything spherical. So we separate balls from tubes.”
The special soot is typically made by vaporizing carbon off graphite rods inside a chamber. As the carbon vapor cools on the chamber walls, much of it condenses into fullerenes, but rare fullertubes also form, sprinkled like gems in a mountain of slag. Stevenson’s magic trick relies on water-soluble molecules known as amines. These are attracted to places where hexagonal arrangements of carbon atoms attach to pentagonal arrangements — intersections that appear all over fullerenes. Nanotubes, on the other hand, are unattractive to amines because they only feature hexagons, and fullertubes are partially protected from amines by their nanotube midsections. So while amines bond to fullerenes, making them soluble in water, unreacted fullertubes remain insoluble; Stevenson can simply rinse the fullerenes away, leaving fullertubes behind.
He then runs his fullertube-enriched samples through machines that separate the molecules based on their mass and subtle chemical differences, yielding pure collections of fullertubes with uniform masses, shapes and properties.

The fullertube mining process starts with graphite rods. Stevenson places a rod into a chamber where an electric arc will vaporize the carbon, leading to the formation of fullerenes and fullertubes.
Ruth Yaro for Quanta Magazine
“Steve’s approach is definitely something that is quite fascinating,” said the chemist Ardemis Boghossian of the École Polytechnique Fédérale de Lausanne in Switzerland, who works with nanotubes. “It’s an approach that isn’t conventionally used in our field. … His is a bit more precise.”
Experts say the ability to isolate pure, uniform samples of fullertubes gives the molecules far more allure than they would have otherwise. Fullerenes can also be isolated, but they lack the electrical and optical properties that make fullertubes and nanotubes promising as components in electrical circuitry or light-based sensors. Meanwhile, purity remains only a dream for nanotube researchers, who often work with a jumble of tubes of random lengths and diameters, and even nested tubes within tubes. So could fullertubes overcome the hurdles that have waylaid its cousins?
Whatever Happened to Buckyballs?
In a 1991 article in Scientific American, Curl and Smalley imagined revolutionary applications of buckminsterfullerenes, including new, carbon-based superconductors, electronics and lubricants. “The versatility of bulk C60 seems to grow week by week,” they wrote.
Five years passed. “No practically useful applications have yet been produced,” wrote the Nobel Prize committee in a 1996 press release announcing that Curl, Kroto and Smalley had won the chemistry prize for discovering buckminsterfullerenes, “but this is not to be expected as early as six years after macroscopic quantities of fullerenes became available.”
A quarter-century later, none of the initially hoped-for products have made it to market. The few places where you might run across buckyballs commercially are cosmetics and dietary supplements that tout the molecule’s potential as an antioxidant. Neither product type requires FDA approval, however, and several studies have shown signs of toxicity in buckyballs. (One study seems to support the health benefits, at least in extending the life spans of mice exposed to ionizing radiation; another finds no life-extending benefits in mice.)
Michael Crommie, a physicist at the University of California, Berkeley, sees fullerenes as significant mainly for forging a trail to other carbon crystals. “Because we got buckyballs,” he said, “that led to nanotubes, and that led eventually to graphene.”
Nanotubes have had more scientific and commercial success than fullerenes. You can pick them up at the hardware store, where they’re found in “nano tape” or “gecko tape” that uses the crystals for adhesion in much the same way that lizards’ feet use microscopic hairs. Nanotubes are extraordinarily strong, with the potential to far outperform steel — except that no one has managed to make nanotubes of sufficient length for ultra-strong cabling. Still, nanotubes add strength when mixed into fabric, boat hulls, high-performance car bodies and tennis rackets. They’re also widely used for water filtration and for enhancing the performance of some batteries.
But whereas those applications involve bulk quantities of nanotubes of various lengths and diameters, more groundbreaking applications such as precision nanosensors will require nanotubes that are identical to one another. Two sensors built from different nanotubes, for example, will respond differently to the same stimulus. Electronics need uniform components in order to function in predictable ways.

Carbon soot (top left) produced from the vaporization process goes through a liquid chromatography machine (top right), which yields vials of pure fullertube samples (bottom).
Ruth Yaro for Quanta Magazine
“We can’t really isolate nanotubes,” said Boghossian. “Maybe the person who finds an easy way to isolate pure nanotubes might get a Nobel Prize,” much as Geim and Novoselov won the physics prize not for discovering graphene, but for isolating it.
Researchers like YuHuang Wang at the University of Maryland are developing a way to snip long nanotubes to produce specific lengths — an arduous top-down technique that starts with a mix of nanotubes and transforms them into a collection of identical sections. Other researchers are trying to construct nanotubes from the bottom up, atom by atom, but this approach is faulty and expensive.
Graphene, with its uniform, single-layer sheets, is where Crommie believes the true potential for carbon nanomaterials will be fulfilled. The best route to carbon-based electronic and magnetic devices, in his view, is to trim graphene ribbons into useful shapes — a technique he says has already led to complex electronic devices in the lab.

The discoverers of buckminsterfullerenes (from left), Sean O’Brien, Richard Smalley, Robert Curl, Harry Kroto and James Heath, posed on a lawn at Rice University in Houston in 1985.
Baby Steps for Fullertubes
So what role, if any, might be filled by fullertubes? Because the crystals are uniform and can be either conductors or semiconductors, Stevenson and Dorn imagine that they could potentially be linked together like nano-size Legos to make miniature electronics.
Boghossian inserts nanotubes into cells to study the environment inside. She relies on nanotube fluorescence: The structures absorb one color of light and emit another, and the light change reveals information about cellular conditions. But the fluorescence depends on the structure of the nanotubes, and differences between them make signals harder to interpret. The shortest fullertubes don’t fluoresce, but the longer ones show signs of it. If even longer fullertubes fluoresce more strongly, they could be a boon to research like hers. “I think it will help a lot with the optoelectronic applications,” she said.
Since 2020, according to a search of academic publications, fullerenes have been mentioned in about 22,700 papers. Nanotubes appear in 93,000. A search on graphene turns up over 200,000 citations. For fullertubes, as of this writing, the all-time total number of relevant publications is 94.
More researchers may make the leap to fullertubes over time, Boghossian says, if studies reveal properties resembling those of nanotubes, with the added benefit of precise lengths. Still, she said, “it will take some adaptation, because people have been working on nanotubes [and other carbon forms] their whole lives.”