Global Microbiome Study Gives New View of Shared Health Risks
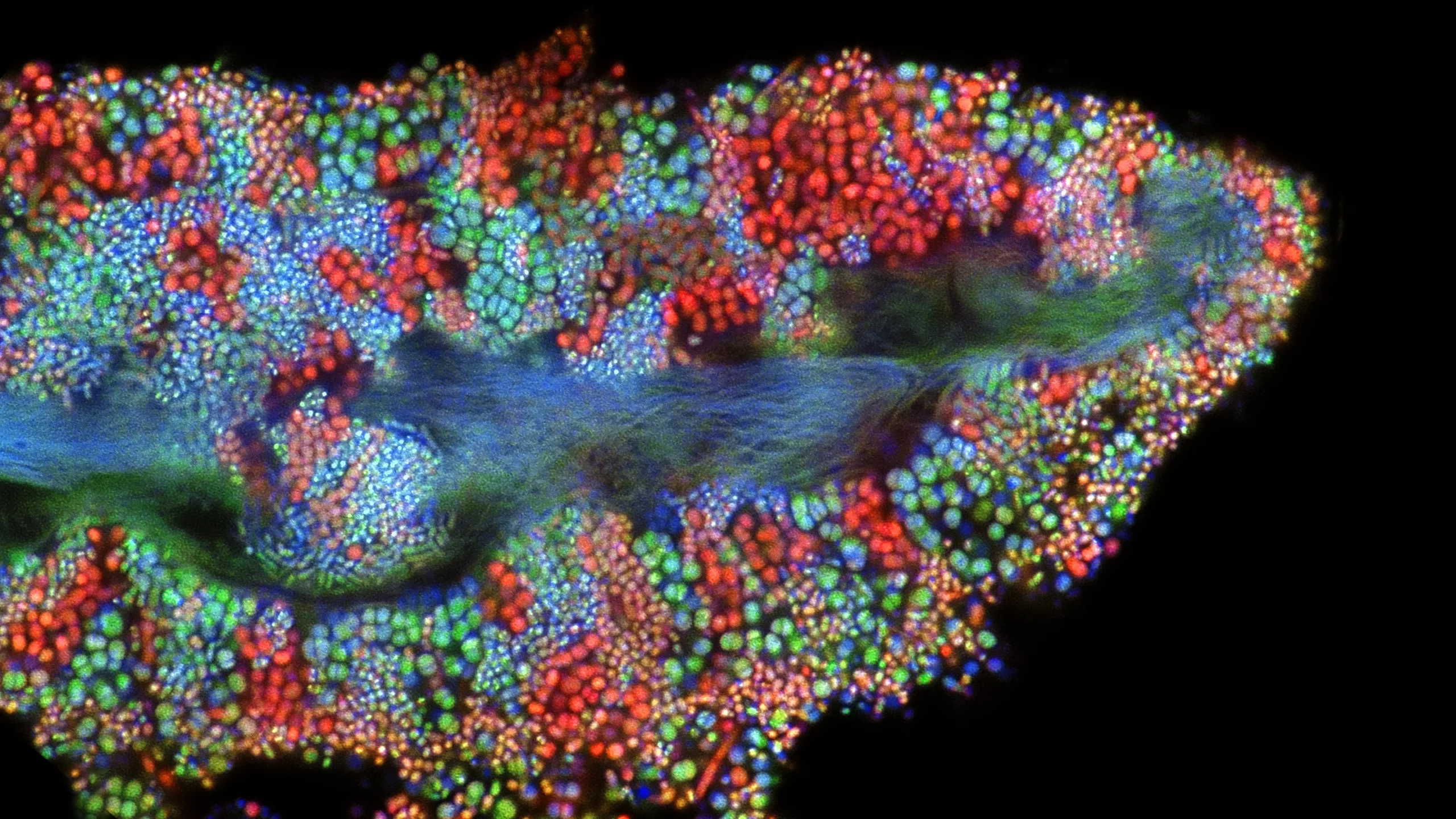
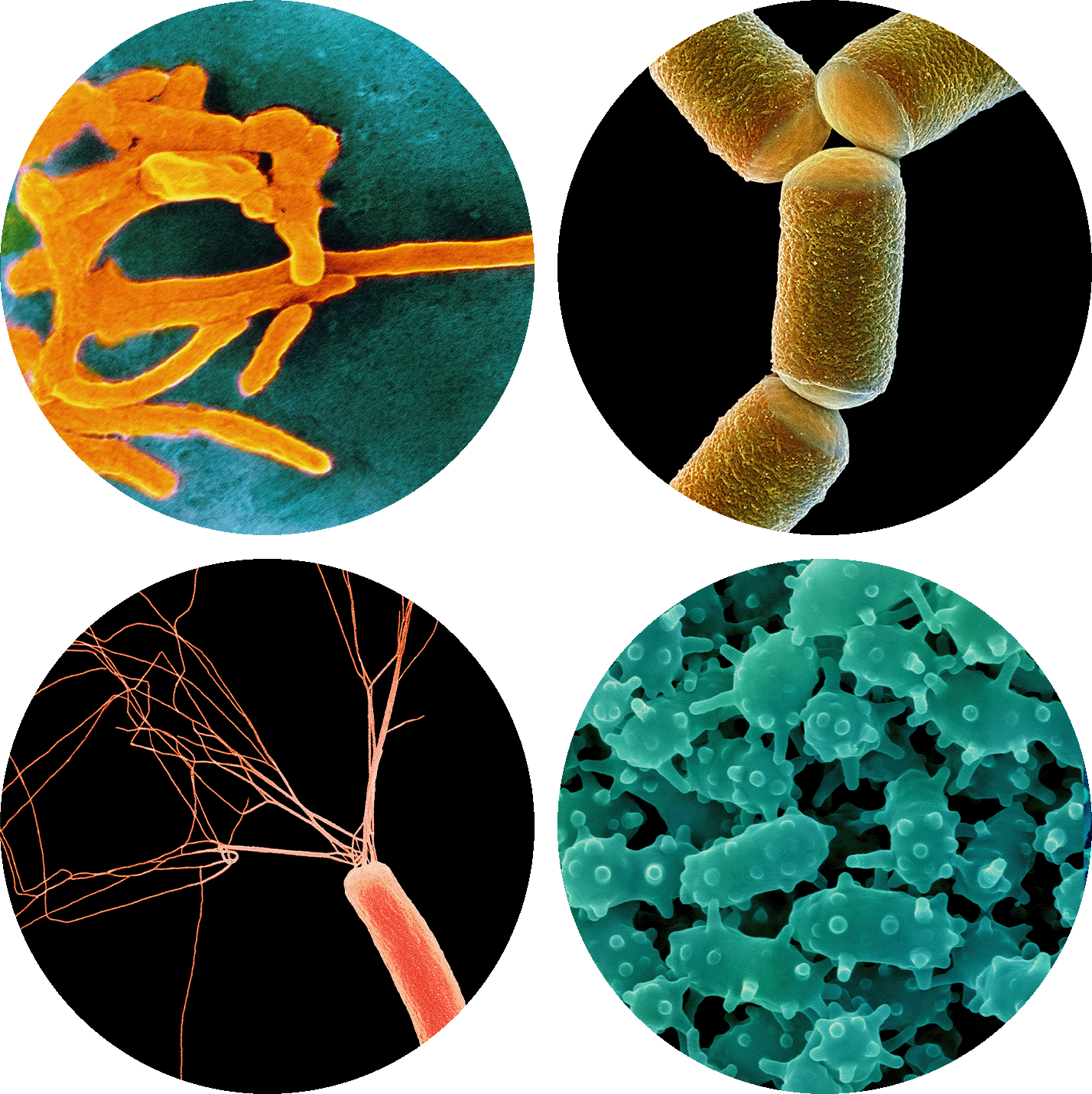
This biofilm shows the diverse and abundant microbes living on a single epithelial cell from the surface of the human tongue. Researchers are starting to understand how often the cells in our microbiomes are acquired from other people.
Tagide deCarvalho
Introduction
Our bodies consist of about 30 trillion human cells, but they also host about 39 trillion microbial cells. These teeming communities of bacteria, viruses, protozoa and fungi in our guts, in our mouths, on our skin and elsewhere — collectively called the human microbiome — don’t only consist of freeloaders and lurking pathogens. Instead, as scientists increasingly appreciate, these microbes form ecosystems essential to our health. A growing body of research aims to understand how disruptions of these delicate systems can rob us of nutrients we need, interfere with the digestion of our food, and possibly trigger afflictions of our bodies and minds.
But we still know so little about our microbiome that we are just starting to answer a much more fundamental question: Where do these microbes come from? Can they spread from other people like a cold virus or a stomach bug?
Now, the largest and most comprehensive analysis of human microbiome transmission has provided some important clues. Research led by genomicists at the University of Trento in Italy have found hints that microbiome organisms hop extensively between people, especially among those who spend a lot of time together. The findings, published in January in Nature, fill important gaps in our understanding of how people assemble their microbiomes and reformulate them throughout their lives.
Other scientists have applauded the study. Jose Clemente Litran, an associate professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, hailed the work as “outstanding” and said it provided the first clear measure of how much sharing to expect among family members or those who live together.
The study also fuels intriguing speculations about whether microbes can raise or lower our risks for diseases likes diabetes or cancer — and thereby bring a transmissible dimension to illnesses that are not usually considered contagious. For Brett Finlay, a professor of microbiology at the University of British Columbia who wrote a commentary for Science in 2020 about that possibility, the findings “put the final nail in the coffin that noncommunicable diseases maybe shouldn’t be called that.”
Unfathomable Diversity
Microbiomes are like fingerprints: so diverse that no two people can have identical ones. They’re also incredibly dynamic — growing, shrinking and evolving so much throughout a person’s lifetime that a baby’s microbiome will look drastically different by the time they grow up. A handful of microbial species are found in more than 90% of people in westernized societies, but most species are found in 20% to 90% of people. (Even Escherichia coli, which is probably the only intestinal bacterium most people could name, falls short of 90% frequency.) Studies suggest that non-westernized societies have an even greater diversity of microbes and more variable microbiomes.
Within a population, any two randomly chosen individuals usually have less than half of their microbiome species in common — on average, the overlap in the microbial makeup of the gut is between 30% and 35%. Microbiologists debate whether there is a “core” set of microbial species that all healthy people have, but if it exists, it’s probably a single-digit percentage of the total.
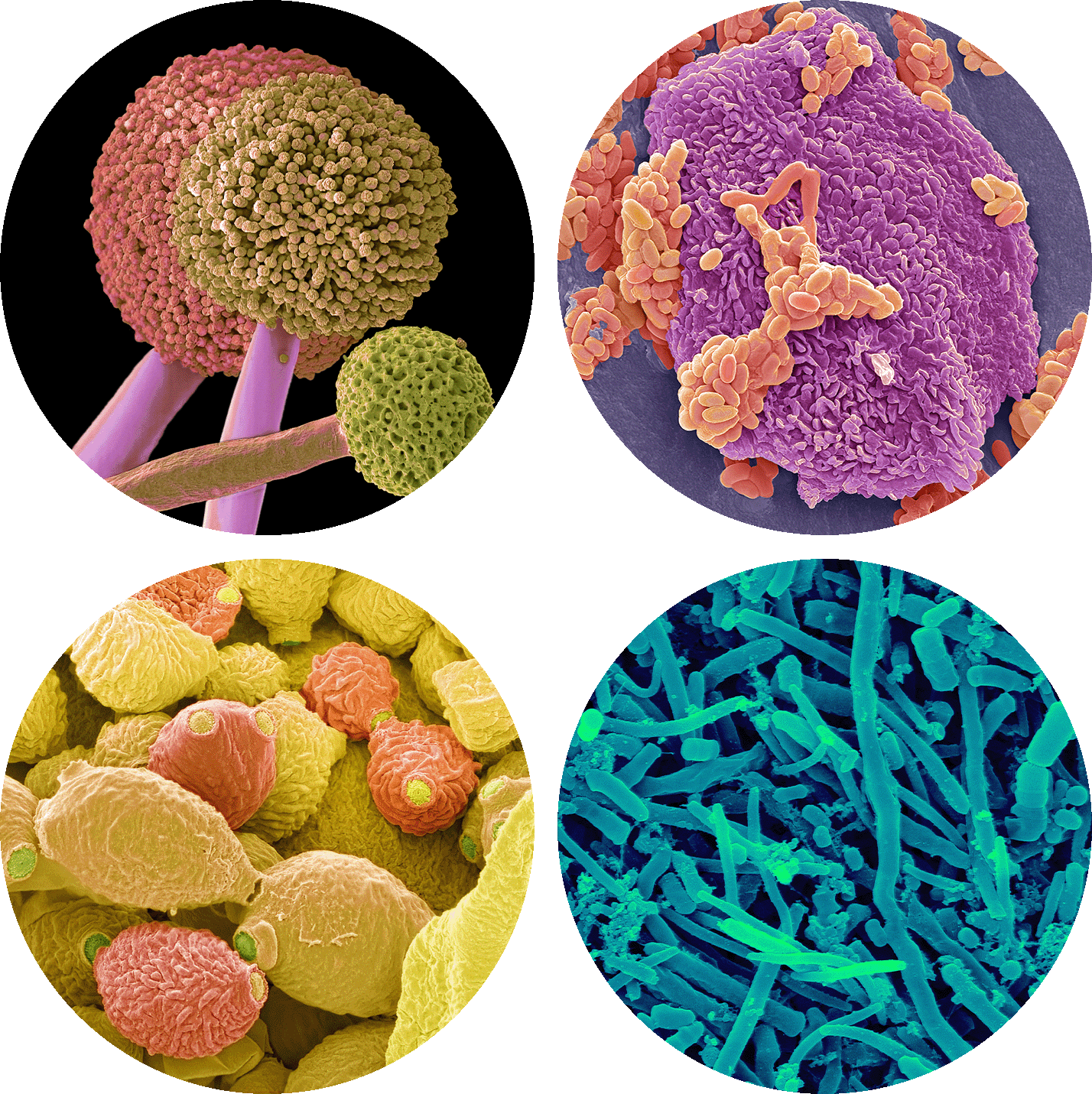
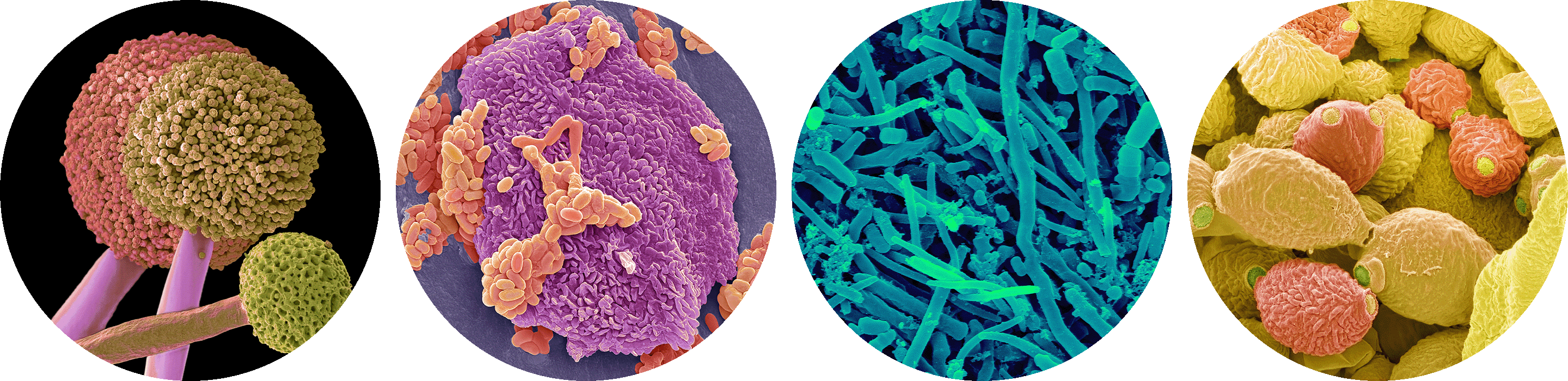
Our oral cavity is home to diverse microorganisms, such as the fungus Aspergillus (at left) and these three samples of bacteria collected from the lips and between the teeth.
Steve Gschmeissner and
Dennis Kunkel Microscopy/Science Source
Determining how often microbes pass between people, however, is a much more formidable problem than looking for species. A single species can consist of many different strains, or genetic variants. Researchers therefore need to be able to identify individual strains by looking at the genes in microbiome samples. And in a human microbiome, between 2 million and 20 million unique microbial genes may be present, with the microbes constantly reshuffling their genes, mutating and evolving.
This is why learning how the multitudes of cells in the microbiome spread is “much more difficult than learning how to trace the spread of one pathogen,” said Mireia Valles-Colomer, a postdoctoral fellow at the University of Trento and the first author on the new study. Until recently, tracing strains through a population was impossible.
In 2010, when Nicola Segata first began analyzing massive genetic data sets for the Human Microbiome Project as a postdoc at Harvard University, the available tools lacked the resolution needed to pinpoint which species were in people’s microbiomes. They could identify the general taxonomic group that a microorganism belonged to, but that was like narrowing down someone’s location to the U.S. Midwest.
Over the next few years, various laboratories found evidence that social interaction and living in proximity affected the microbiomes of primates and mice. Studies of humans conducted on relatively isolated populations in Papua New Guinea and elsewhere also found signatures of microbial sharing. Some even found traces of possible transmission from pets. But because of the limitations of those studies, it wasn’t clear how much transmission was happening and whether it happened everywhere to the same degree.
This changed after Segata established his lab at the University of Trento in 2013. He and his team began to create and refine metagenomics tools that could distinguish between strains of the same species, which made it possible to study microbiome transmission in more detail.
Segata started probing this question in 2018 by analyzing the microbes of mothers and their infants. His group’s findings and several other studies confirmed earlier suspicions that there is a massive amount of transmission from mother to baby, such that the mother is “imprinting the microbiome at birth,” Segata said. Recent work has shown that mothers continue to mold the microbiomes of their infants over the next few years.
But the diversity of the microbiome changes significantly between childhood and adulthood, so this early inheritance from mothers “is not explaining the microbes we are seeing in the adults,” Segata said. In follow-up experiments, the researchers largely ruled out the possibility that the new microbes came from the food people ate, because those microbes weren’t able to colonize the gut very well.
So “it has to be transmission,” Segata said. “It has to be that what we have in the gut is coming from the gut of other individuals.”
Sharing With Family and Friends
For the new global analysis of microbiomes, Segata, Valles-Colomer and their colleagues honed their tools enough to recognize previously unknown species and different strains of the same species. Using these tools, they examined more than 9,700 samples of stool and saliva from 20 countries on five continents, representing communities with very diverse lifestyles and covering the full range of the human life span and many different living arrangements. They traced more than 800,000 strains of microbes between families, roommates, neighbors and villages and calculated what percentage of shared species were the same strain.
As they expected, they found that the most sharing of strains happened between mothers and infants in the first year of life — about 50% of the shared species found in the infants’ guts were strains that spread from the mother. The mother’s influence diminished with time — slipping from 27% at age 3 to 14% by age 30 — but didn’t disappear. Some elderly people in China were shown to still share strains with their surviving centenarian mothers.

The researchers Mireia Valles-Colomer and Nicola Segata of the University of Trento in Italy recently completed a massive analysis of human microbiome transmission around the world —the most comprehensive such study ever performed.
University of Trento
For Veena Taneja, an immunologist at the Mayo Clinic who was not involved in the study, one of the more surprising tidbits in the findings was that although infants born vaginally shared more strains with their mothers than infants born by C-section did, this difference vanished by three years of age. “People make a big deal out of it” that babies born via C-section might be more at risk for certain diseases, she said. But the findings suggest that maybe it “should not be a big thing.”
(That view was corroborated by a new study published this month in Cell Host & Microbe. It found that babies born via C-section received less of their mother’s microbiomes than babies born vaginally, but that they didn’t miss out because they received more microbes from breast milk.)
As we get older, a sizable portion of our microbiomes continues to come from the people we live with or near. Unsurprisingly, the study by Segata and colleagues found that spouses and other physically intimate partners shared a lot of microbes: 13% of the gut species they shared were of the same strain, as were 38% of their shared oral species.
But people who lived together platonically weren’t far behind, at 12% for shared gut species and 32% for shared oral species. That’s because, as Segata, Valles-Colomer and their team found, the single most important determinant of transmission was time spent together. People living under one roof shared the most strains, but even people living in the same village tended to have more strains in common than people separated by greater distances. The frequency of strain sharing was consistent across different societies, but the team did confirm previous findings that people in non-westernized countries tend to have more diverse microbiomes.
The researchers also found that strains held in common could be lost over time. Twins growing up together had about a 30% strain-sharing level that dropped to about 10% after 30 years of living apart.
Segata thinks it’s likely that most of the other strains of shared species also come from other people — primarily from close contacts like friends or co-workers, but maybe also from people we encounter far more briefly and casually. (Pets, however, are probably not big contributors: Segata said that animals mostly harbor microbial species that don’t typically colonize or persist in us.)
The findings are the strongest evidence to date that we share parts of our microbiomes with the people we spend the most time with. The fact that the authors were able to see this pattern of transmission across the globe, and not just in a single population, was “striking,” said Ilana Brito, an associate professor in biomedical engineering at Cornell University. These data sets are extremely noisy, with many mutations happening across these different organisms, she added. But the team successfully uncovered “the signal across the noise.”
It’s not clear how microbiome organisms spread between people. Kissing and sex explain some of it, but microbes could also be transmitted through droplets spewed by coughs and sneezes, or they could be picked up from contaminated surfaces. There’s also still a lot to learn about which microbes are more easily spread than others. Answering that question is critical for understanding the implications of the idea that microbiome organisms can spread.
Spreading Health or Disease
Now that the extent of sharing has revealed the patterns of distribution of unique microbes, we can examine what happens in disease. “In that sense, I think this work is really fundamental,” Clemente said.
Some diseases that aren’t usually considered contagious could have an overlooked communicable aspect. Studies have found that many people with diseases that don’t spread person to person have microbiomes that seem to be “screwed up,” Finlay said.
Some E. coli strains, for example, may release toxins that could increase the risk of cancer. People with certain colorectal cancers whose microbiomes contain more of a Fusobacterium species tend to have a worse prognosis and worse outcomes with treatment. Gut microbes that affect glucose and insulin levels in the body have been tied to obesity and conditions like metabolic syndrome and even Type 2 diabetes. An unbalanced gut microbiome has been linked to neurodegeneration, and it’s theorized that it might play a role in brain conditions like Alzheimer’s disease.
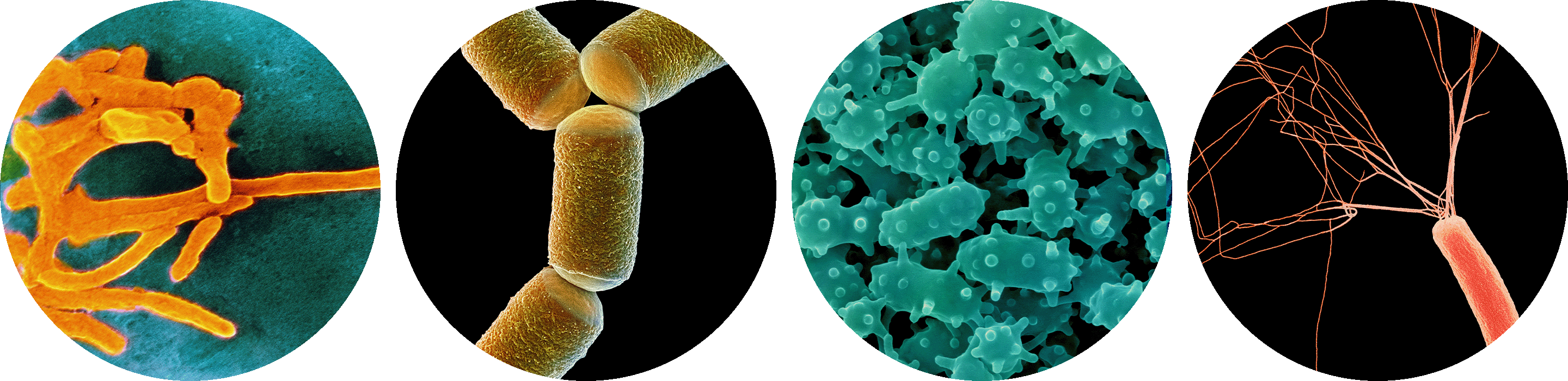
Among the countless species of organisms that live in the human gut microbiome are the bacterium Fusobacterium nucleatum, the archaeon Methanobrevibacter smithii, the bacterium Verrucomicrobium spinosum and the bacterium Escherichia coli.
CNRI, Steve Gschmeissner,
Dennis Kunkel Microscopy/Science Source
“If these diseases are at least partially dependent on the microbiome, and then the microbiome is at least partially transmissible, then these diseases become at least partially transmissible,” Segata said.
But “understanding the amount to which a certain microbiome contributes to [disease] risk, that’s the hard question,” Clemente said. Even most studies that find such associations can’t tease apart whether the microbes cause the disease or simply find it easier to colonize a person at risk for the disease.
If “bad” microbes that raise the risk of noncommunicable health problems can be transmitted between people, then in theory “good” microbes that lower those risks can be as well. Some studies suggest that microbes can be protective, especially in early life, against conditions like asthma and allergies. Deliberately sharing pieces of healthy microbiomes, such as through fecal transplants, has proved astonishingly successful in treating certain diseases and infections like that of the bacteria Clostridium difficile.
We evolved to maintain our microbial populations because we greatly benefit from them, said Jens Walter, a professor of ecology, food and the microbiome at University College Cork and the APC Microbiome Ireland. That’s why Walter is unconvinced by the hypothesis that our shared microbes might be causing diseases and is more drawn to the opposite idea, sometimes called the “old friends” or hygiene hypothesis. It proposes that throughout evolution, our microbiomes may have helped to train the responses of our immune system. The modern increase in the use of antibiotics and antiseptics and our greater general cleanliness could therefore be altering the makeup of the microbiome and creating more health vulnerabilities for us.
Compared to a century ago, “we are definitely not spreading microbes more readily in today’s world,” Walter said. Inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis and Type 1 diabetes — all of which are considered immunological disorders rather than communicable diseases — are more prevalent in westernized societies that tend to use antibiotics and antiseptics extensively.
The beneficial or detrimental effects of sharing could depend on which species and strains are shared, which is still a bit of black box. We should also consider, Brito said, that it might not be individual organisms in our microbiome that affect our health but rather communities of them that get transmitted together. Certain organisms might matter more in one community context than another.
Segata, Valles-Colomer and their team analyzed only healthy individuals in their study, but in their ongoing research, they are applying their metagenomic tools to data sets from people with diseases to see if those findings illuminate the connections between health and microbiomes.
They are also currently sampling data from three daycare centers — from infants and their parents, siblings, pets and teachers. The researchers are hoping to figure out how the microbes are transmitted and how long it takes for specific gut and oral microbes to jump between people.
Tracking the spread of microbiome organisms was neglected for a long time because “we did not think it would have so much influence on our health,” Valles-Colomer said. Now that we have the techniques to probe the microbiome, “we see it associated with virtually any disease.”
Editor’s note: Research by Segata and his group has received funding from the Simons Foundation, which also funds this editorially independent magazine. Simons Foundation funding decisions have no influence on our coverage.