How Animals Color Themselves With Nanoscale Structures

Like many animals, the blue-winged leafbird of Southeast Asia gets its iridescent hues from structural colors — materials with nanometer-scale features that diffract and reflect specific wavelengths of light.
Wulong Tommy
Introduction
Peacocks, panther chameleons, scarlet macaws, clown fish, toucans, blue-ringed octopuses and so many more: The animal kingdom has countless denizens with extraordinarily colorful beauty. But in many cases, scientists know much more about how the animals use their colors than how they make them. New work continues to reveal those secrets, which often depend on the fantastically precise self-assembly of minuscule features in and on the feathers, scales, hair and skin — a fact that makes the answers intensely interesting to soft matter physicists and engineers in the photonics industry.
Many of the colors seen in nature, particularly in the plant kingdom, are produced by pigments, which reflect a portion of the light spectrum while absorbing the rest. Green pigments like chlorophyll reflect the green part of the spectrum but absorb the longer red and yellow wavelengths as well as the shorter blue ones. Which specific wavelengths get reflected or absorbed depends on the pigment’s molecular makeup and the exact distances between the atoms in its molecular structures.
Because plants are masters of biochemical synthesis, their cells can concoct many types of pigments, but animals by and large have lost the metabolic pathways to make most of them. Melanin, the predominant pigment in animals, is either brown (eumelanin) or reddish yellow (pheomelanin) — a rather limited palette. To make the richer rainbow of colors they need for decorating and disguising themselves, courting mates and warding off predators, animals can often obtain the needed pigments from their diet. Birds’ bright reds and yellows, for instance, mostly come from carotenoid pigments in their food.
The blue end of the spectrum, however, represents a different challenge because few blue pigments are available to eat in nature. Yet blue jays, neon tetras, poison dart frogs and many other animals found a solution that doesn’t rely on pigments, evolving optical tricks to make blues (and some greens) a different way. They make what are called structural colors.
Structural colors act like filters that allow only some wavelengths to pass through. Their specific photonic mechanisms vary from species to species, but they work because nanometer-scale structures in their materials are comparable to wavelengths of light. The structures diffract colors of light differently and set up interference effects.
“It’s about having multiple tiny structures that scatter light, and then having those scattered waves interact — that interaction will reinforce some colors and eliminate the others,” explained Richard Prum, an expert on bird-feather coloration at Yale University.

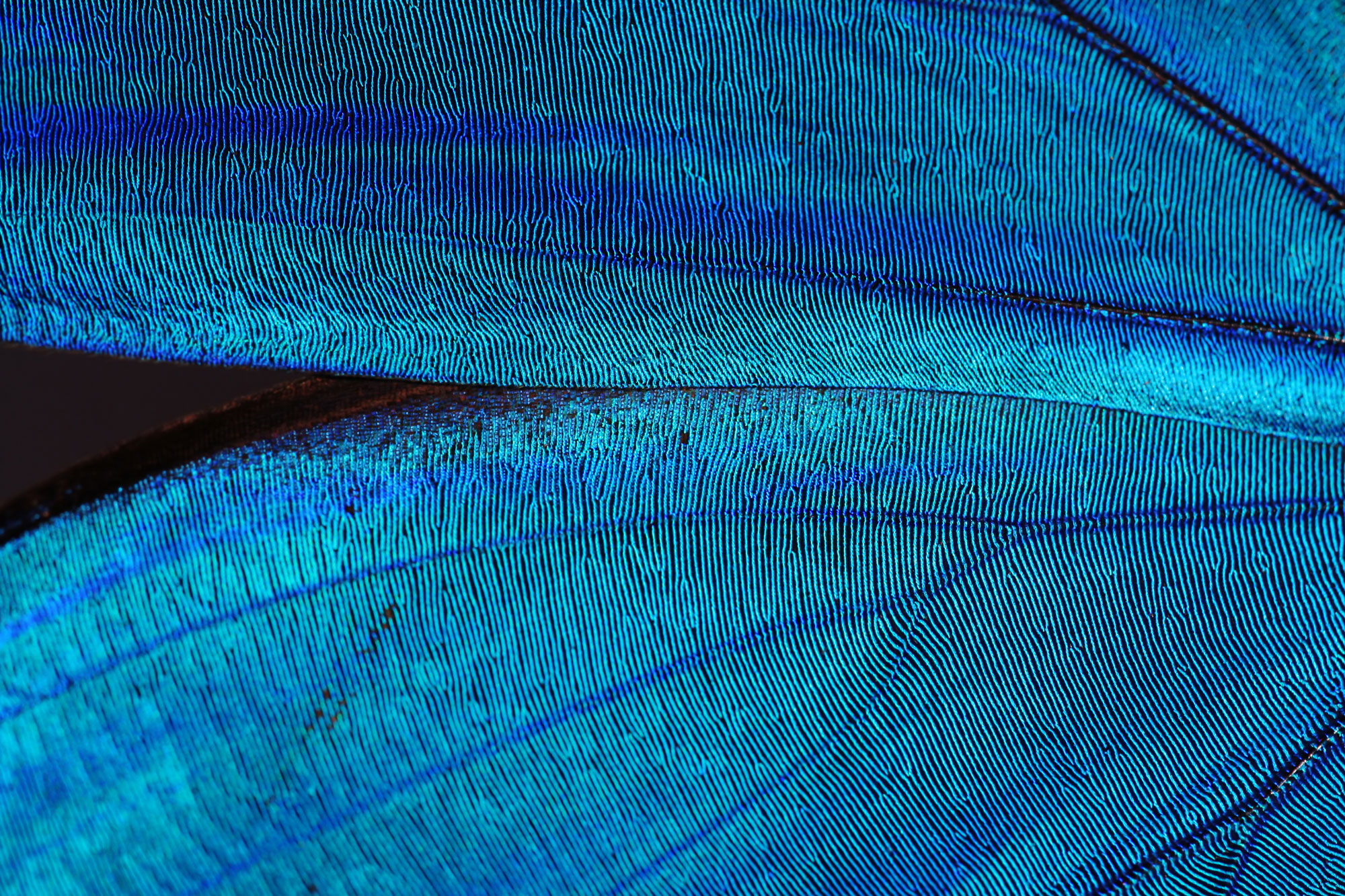
The stunning blue iridescence of the blue morpho butterfly results from the way that structures in its wing scales diffract and reflect blue light while absorbing other parts of the spectrum.
The stunning blue iridescence of the blue morpho butterfly (left) results from the way that structures in its wing scales (right) diffract and reflect blue light while absorbing other parts of the spectrum.
This structural approach to coloration has the advantage of adaptability: “It doesn’t matter what material you make it with, as long as it is kind of transparent,” explained Mathias Kolle, who studies biologically inspired optical materials at the Massachusetts Institute of Technology.
Structural colors also often have the sparkly visual appeal of iridescence. Because light reflecting from the top of a structural-color layer can be out of phase with light reflecting from the bottom, the color can seem to brighten or shift in hue when viewed from different angles. That effect is striking in the bright blue of morpho butterflies, for example. The scales on a morpho butterfly wing are sculpted with minute grooves lined with treelike protrusions that diffract and reflect light waves, causing them to interfere with each other in a way that produces iridescent blue.
In a 2015 study, Kolle and his colleagues reported their discovery of how a mollusk, the blue-rayed limpet, generates the distinctive bright blue stripes on its shell. Layers of transparent calcium carbonate crystals in the shell are arrayed as multiple microscopic sheets, with each layer diffracting and reflecting a sliver of light. The diffracted light waves interact with each other; depending on the thickness of each layer and the wavelength of the light, the waves either add up or cancel out. By getting the thickness of the layers just right (100 nanometers), the limpet makes all the wavelengths except blue ones cancel one another out.



The distinctive blue stripes on the blue-rayed limpet (top) reflect out of a layered arrangement of transparent calcium carbonate crystals in the shell.
The distinctive blue stripes on the blue-rayed limpet (left) reflect out of a layered arrangement of transparent calcium carbonate crystals in the shell (right).
johndal; Ling Li
Other animals exploit similar phenomena in their structural colors. For instance, one trick behind the color-change artistry of octopuses and other cephalopods is that some of the chromatophore cells in their skin contain layers of proteins called reflectins that can quickly shift from an ordered to a disordered state. By thickening and thinning those layers, the animals can reflect different wavelengths and alter the colors they show the world.
Unlike the octopus, though, the limpet can’t change the shape of its layers after they are laid down. How the limpet builds the layered structure with such precision is a mystery. “The material science dynamics behind it is mind-blowingly not-understood,” Kolle said. But work by Prum, Vinod Saranathan of Yale-NUS College at the National University of Singapore and others in recent years has made progress on understanding how some birds produce the structural colors behind their brilliant blue plumage: a process of phase separation.
At high magnification, the colored barbs (filaments) of feathers have a foamy structure, with small, uniform spheres of air suspended in beta-keratin protein. The light scattering off each air bubble interacts with the light bouncing off neighboring bubbles. “And because they are just the right size to do this, they make a blue color, or a turquoise color, or an ultraviolet color,” Prum said.
Studies indicate that inside the cells of a developing bird feather, the beta-keratin starts out distributed in the watery cytoplasm. Chemical changes in the cell cause the beta-keratin and the water to spontaneously separate, creating spherical water droplets within a matrix of polymerized protein. After the cell dies, the water evaporates and the spaces fill with air, leaving a miniature ball pit of air bubbles that reflects light at just the right wavelength.
Prum likens the process to opening a bottle of beer. “Suddenly you get a condensation — the dissolved carbon dioxide forms a bubble, and the bubble grows to a certain size and then floats up,” he said. “This stuff looks just like the head on a beer.”
In the blue feathers of blue jays and most other birds, these bubbles are disordered. But at least one species, the blue-winged leafbird of Southeast Asia, gets the shimmery blue of its shoulder feathers from perfectly ordered bubble crystals, as Saranathan, Prum and their colleagues reported in the Proceedings of the National Academy of Sciences on June 8. The researchers discovered these gyroid crystals when they put the leafbird feathers under a powerful X-ray beamline at Argonne National Laboratory.
The gyroid, a continuous minimal surface that forms a highly periodic structure, is in a sense the reverse of a sphere: While a sphere has uniform positive curvature, the gyroid is a saddle-shaped object with uniform negative curvature. One of its special features is that it splits space into two labyrinths of tunnel systems, separated by a membrane, that perfectly mirror each other. When both sets of tunnels are filled with fluid inside a live cell, the structure is known as a double gyroid; when only one set of tunnels is filled, the structure is a single gyroid.
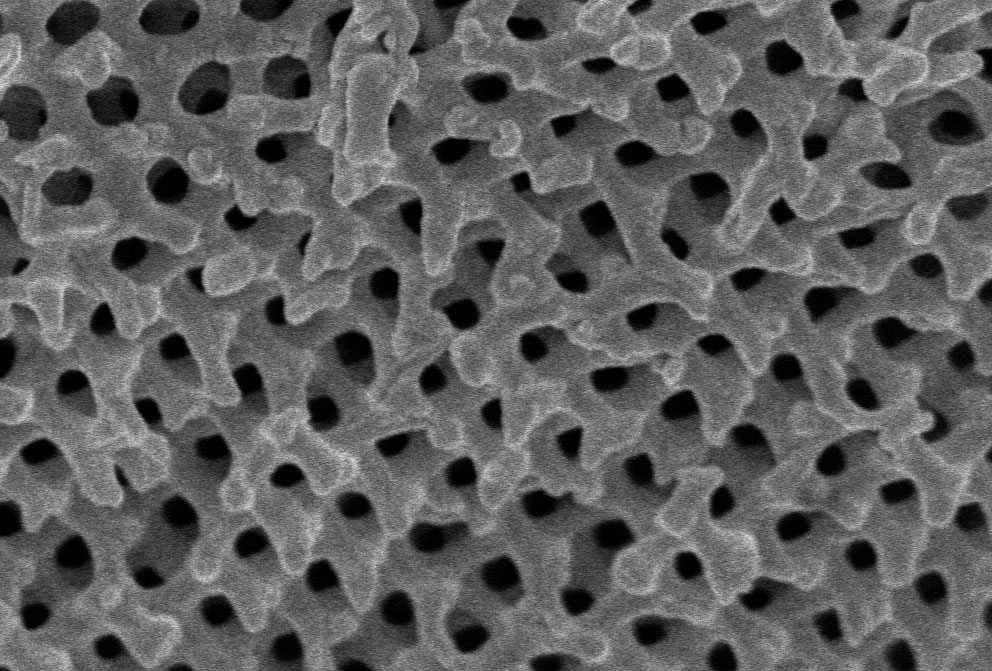
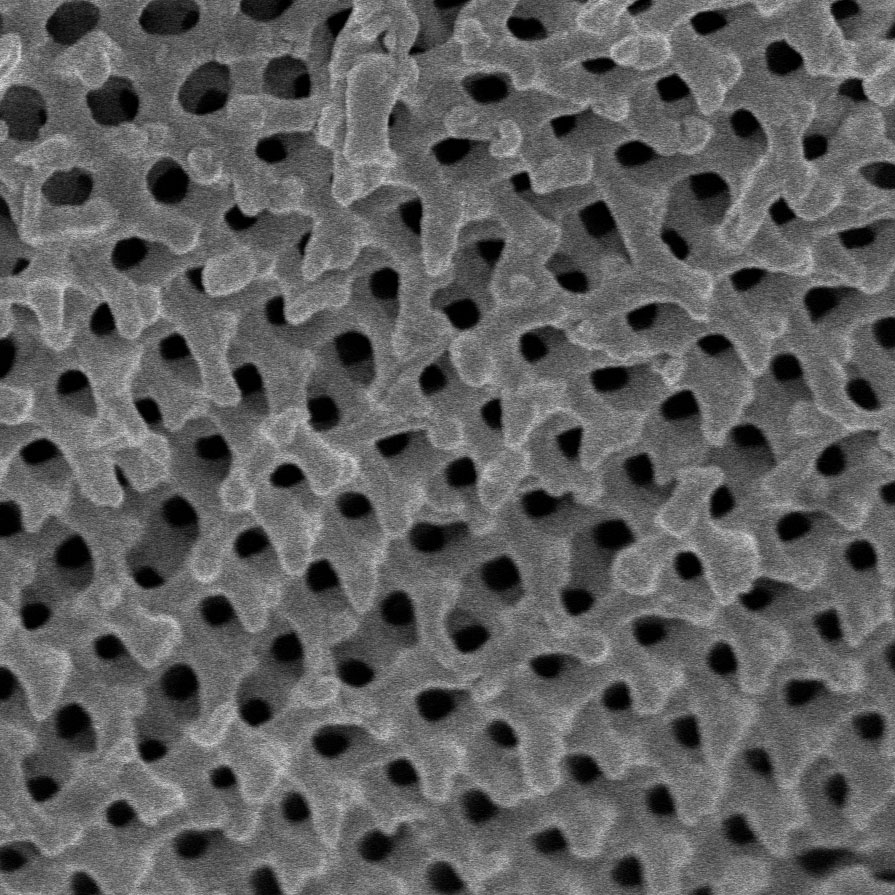
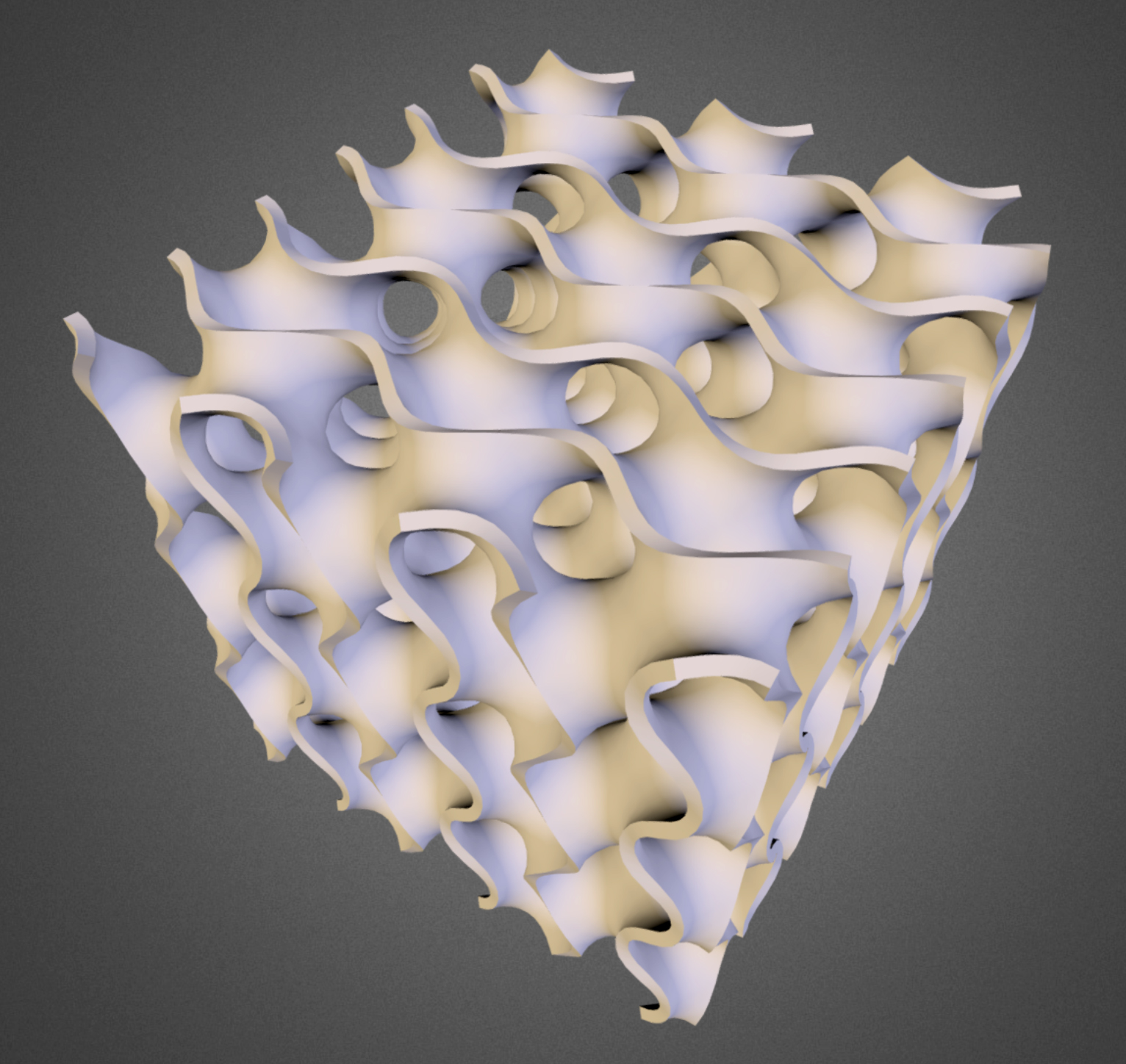
The periodic arrangement of nanoscale bubbles in leafbird feathers (top) are produced by the self-organized growth of a gyroid structure (right) within the barbs of the feathers.
The periodic arrangement of nanoscale bubbles in leafbird feathers (left) are produced by the self-organized growth of a gyroid structure (right) within the barbs of the feathers.
Vinodkumar Saranathan; Mersus
The leafbird’s single gyroid crystals exhibit the same optical property as the limpet’s layers. “You have locally periodic changes of refractive index,” or a periodic arrangement of different light-scattering materials, explained Bodo Wilts, a soft matter physicist at the Adolphe Merkle Institute in Fribourg, Switzerland.
Single gyroids have previously only been seen in nature in some butterfly scales, as reported in 2010 by Saranathan, Prum and their colleagues. Gerd Schröder-Turk, who studies biophotonic materials at Murdoch University in Australia, and his colleagues have shown when these scales are developing, the endoplasmic reticulum membrane in the scale cells forms a sheet with fluid on either side, creating a double gyroid. One of the tunnels then fills with chitin and solidifies. When the cells die, they leave behind a single gyroid.
Researchers thought that this molding or templating process was the only way single gyroids could form in nature. Instead, evidence points to the leafbird making its gyroids the same way that its close relative the blue jay makes its disordered ball pits of bubbles — by phase separation. It’s something that could not have been predicted based on existing theory in soft matter physics, Saranathan and Prum say.
The discovery suggests that crystals like this can self-assemble, which is encouraging for engineers looking for better ways to make materials for photonic applications. To transmit blue light more efficiently, for example, a fiber-optic cable could be lined with the kind of blue-reflecting material found in the leafbird so that no blue photons can escape.
“All the optical fibers that are now laboriously made with precision engineering — birds do it by self-assembly,” Prum said. Learning how to grow self-assembled photonic devices “would be a real cost savings.”
Kolle agrees. Last year in Nature Photonics, he and his team described an improved method of dark-field imaging microscopy that uses a material inspired from the wing scales of Papilio butterflies. Now he is working with a student to observe how the nanoscale grooves in the wing scales of the painted lady butterfly are sculpted. Understanding the process in this species should unlock how the basic scale architecture develops in most butterflies.“I hope that there are biomechanical principles … that we can apply to make these materials in a completely different materials system,” he said.