How Microbiomes Affect Fear

Researchers are finding evidence that microbiomes can influence the fear responses of their hosts, possibly by releasing compounds that affect the brain’s neuroanatomy and function.
Eoin Ryan for Quanta Magazine
Introduction
Our brains may seem physically far removed from our guts, but in recent years, research has strongly suggested that the vast communities of microbes concentrated in our digestive tract open lines of communication between the two. The intestinal microbiome has been shown to influence cognition and emotion, affecting moods and the state of psychiatric disorders, and even information processing. But how it could do so has been elusive.
Until recently, studies of the gut-brain relationship have mostly shown only correlations between the state of the microbiome and operations in the brain. But new findings are digging deeper, building on research that demonstrates the microbiome’s involvement in responses to stress. Focusing on fear, and specifically on how fear fades over time, researchers have now tracked how behavior differs in mice with diminished microbiomes. They identified differences in cell wiring, brain activity and gene expression, and they pinpointed a brief window after birth when restoring the microbiome could still prevent the adult behavioral deficits. They even tracked four particular compounds that may help to account for these changes. While it may be too early to predict what therapies could arise once we understand this relationship between the microbiome and the brain, these concrete differences substantiate the theory that the two systems are deeply entwined.
Pinning down these mechanisms of interaction with the brain is a central challenge in microbiome research, said Christopher Lowry, an associate professor of integrative physiology at the University of Colorado, Boulder. “They have some tantalizing leads,” he added.
Coco Chu, the new study’s lead author and a postdoctoral associate at Weill Cornell Medicine, was intrigued by the concept that microbes inhabiting our bodies could affect both our feelings and our actions. Several years ago, she set out to examine these interactions in fine-grained detail with the help of psychiatrists, microbiologists, immunologists and scientists from other fields.
The researchers performed classical behavioral training on mice, some of which had been given antibiotics to dramatically diminish their microbiomes and some of which had been raised in isolation so that they had no microbiome at all. All the mice learned equally well to fear the sound of a tone that was followed by an electric shock. When the scientists discontinued the shocks, the ordinary mice gradually learned not to fear the sound. But in the mice with depleted or nonexistent microbiomes, the fear persisted — they remained more likely to freeze at the sound of the tone than the untreated mice did.
Peering inside the medial prefrontal cortex, an area of the outer brain that processes fear responses, the researchers noticed distinct differences in the mice with impoverished microbiomes: Some genes were expressed less. One type of glial cell never developed properly. Spiny protrusions on the neurons associated with learning grew less plentifully and were eliminated more often. One type of cell showed lower levels of neural activity. It’s as if the mice without healthy microbiomes couldn’t learn to be unafraid, and the researchers could see it on a cellular level.
The researchers also set out to learn how the condition of the microbiome in the gut caused these changes. One possibility was that microbes send signals to the brain through the long vagus nerve, which carries sensations from the digestive tract to the brain stem. But snipping the vagus didn’t alter the behavior of the mice. It also seemed possible that the microbiomes might stir up responses in the immune system that affect the brain, but the numbers and proportions of immune cells in all the mice were similar.
But the researchers did pinpoint four metabolic compounds with neurological effects that were far less common in the blood serum, cerebrospinal fluid and stool of the mice with impaired microbiomes. Some of the compounds were already linked to neurological disorders in humans. The team speculated that the microbiome might produce certain substances in abundance, with some molecules making their way into the brain, according to the microbiologist David Artis, the director of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine and the senior author on the study.
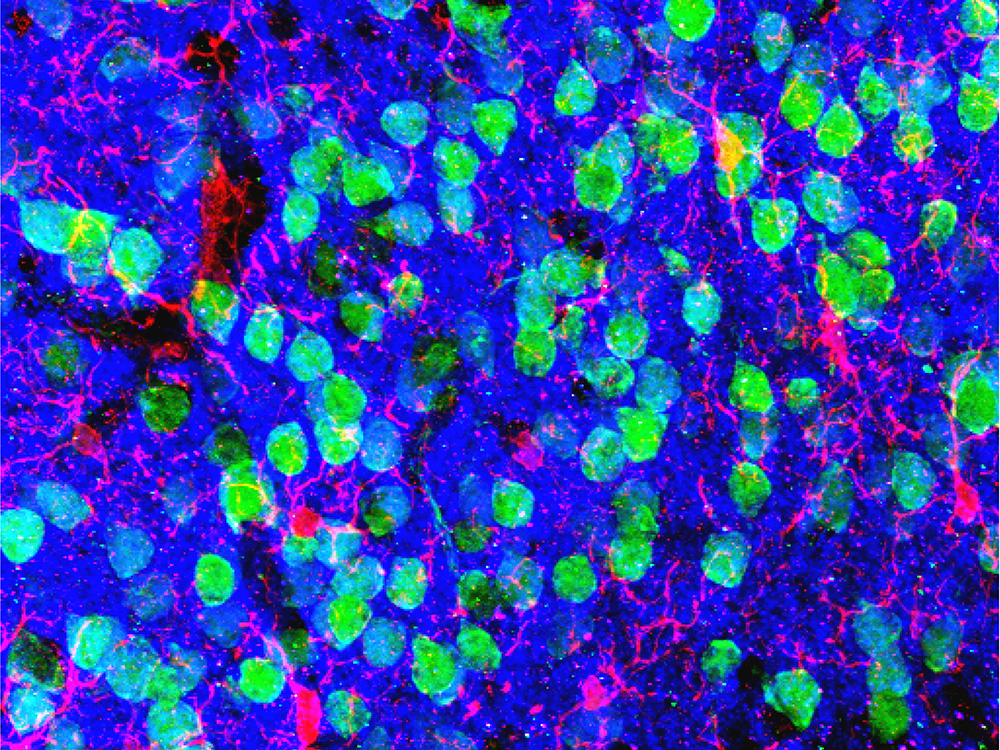
The medial prefrontal cortex, an area near the front of the brain, is important in the extinction, or “unlearning,” of fear responses. In this micrograph of the region, neurons appear green and microglial cells are red. Researchers found abnormalities in these cells in mice that had depleted microbiomes.
Image courtesy of Drs. Christopher Parkhurst and David Artis (WCM)
In many laboratories, there’s a growing interest in tracking specific bacterial substances that are involved in nervous system signaling, said Melanie Gareau, an associate professor of anatomy, physiology and cell biology at the University of California, Davis. Numerous metabolites and pathways are probably involved in such processes.
Research on other disorders like depression has also pointed to the involvement of particular compounds created by microbes, but there’s still no consensus on which ones contribute to any condition, said Emeran Mayer, a professor of medicine and director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience at the University of California, Los Angeles. And although the intestinal microbiome is clearly altered in many people with brain conditions, it’s often unclear if that change is a cause or an effect, he said. Differences in the microbiome might give rise to neurological problems, but the conditions could also change the microbiome.
There’s disagreement within the field not just about the consequences of diseased microbiomes, but also about healthy ones. “For a long time, we’ve been focused on this idea that we could identify specific types of bacteria that provide either risk or resilience to stress-related disorders, and it may be that it doesn’t have to be a particular microbe,” Lowry said. Even in healthy people, microbiomes vary widely. Particular microbes might not matter if a microbiome has enough diversity — just as there are many kinds of thriving forests, and one individual type of tree may not be necessary.
Still, the study of microbial effects on the nervous system is a young field, and there is even uncertainty around what the effects are. Previous experiments reached inconsistent or contradictory conclusions about whether microbiome changes helped animals to unlearn fear responses. What gives extra weight to the findings from Chu and her colleagues is that they can point to evidence for a specific mechanism causing the behavior they observed. Animal studies like this one are especially helpful in cementing a clear connection between the nervous system and the microbiome, even if they don’t point to treatments for humans, said Kirsten Tillisch, a professor of medicine at the David Geffen School of Medicine at UCLA. “The way that humans process emotion, physical sensation and cognition in the brain is just so different than in animals that it’s just very difficult to translate,” she said.
In theory, the presence of certain microbial substances might help predict who is most vulnerable to disorders like post-traumatic stress disorder. Experiments like these could even identify pathways of communication between the brain and the microbiome that could be targeted by treatments. “That’s always the big hope from these mouse experiments, that we’re getting close to interventions,” Mayer said, and the studies often generate striking results through rigorous methods. But the operations of the human brain aren’t fully reflected in mice. Moreover, the interactions of the brain and the gut microbiome differ in humans and mice, and diet-driven differences between their respective microbiomes add to the disparity.
For humans, interventions targeting the microbiome might be most effective in infancy and childhood, when the microbiome is still developing and early programming takes place in the brain, Mayer said. In this new research, the scientists saw a specific window of time in infancy when mice needed a typical microbiome to extinguish fear normally when they grew up. Mice that were totally isolated from microbes for their first three weeks were then mixed in with mice that had typical microbiomes. The germ-free mice picked up the microbes of the other mice and developed rich microbiomes, but when they grew up and went through the same fear unlearning experiments, they still showed deficits. At only a few weeks old, they were still too old to learn to extinguish their fear normally.
But when microbiomes were restored in newborn mice, who gained rich microbiomes after they were placed with foster parents, the infant mice grew up to behave normally. In the first few weeks after birth, the microbiome appeared to be critical — an insight that fits smoothly into the larger idea that circuits governing fear sensitivity are impressionable during early life, Tillisch said.
The kind of fear unlearning that the researchers tested is a fundamental skill in an evolutionary sense, Artis said. Knowing what merits fear and adapting when it no longer poses a threat can be crucial to survival. An inability to extinguish fear is also present in PTSD and tied to other brain disorders, so deepening scientific knowledge around the mechanisms that influence this circuitry could illuminate core human behaviors and pave the way for potential therapies.
On an evolutionary timescale, human microbiomes have changed as more people have come to live in cities, and brain disorders have become increasingly prominent. The swarms of microbes inhabiting each of us have evolved with our species, and it’s vital that we understand how they impact both physical and mental health, Lowry said. Our environments may affect our nervous systems by way of the microbiome, adding new layers of complexity to the study of health and disease in the brain.