Human Brains Are Hard to Study. He Grows Useful Substitutes.

For years, the neuroscientist Sergiu Paşca has been a leader in the use of brain organoids in studies of human neurological disorders. His latest work, in which human cells become working parts of rat brains, carries that effort to new heights.
Rachel Bujalski for Quanta Magazine
Introduction
The mission statement on the homepage of the Stanford University laboratory of Sergiu Paşca is at once simple and spectacularly ambitious. His group “seeks to understand the rules that govern the molecular and cellular steps underlying the assembly of the human nervous system and the molecular mechanisms that lead to neurological and psychiatric disease.”
Paşca’s chosen route to this goal uses an advanced form of stem cell technology. First, he genetically reprograms skin cells from autistic people and patients with disorders like schizophrenia to become versatile stem cells; then he induces the cells to settle into a more defined state as neural tissue in a laboratory dish. By observing how these cells function or malfunction, Paşca, a professor of psychiatry and behavioral sciences at Stanford and a director at the Wu Tsai Neurosciences Institute, gains insights into what makes the brains of people with neurological conditions different.
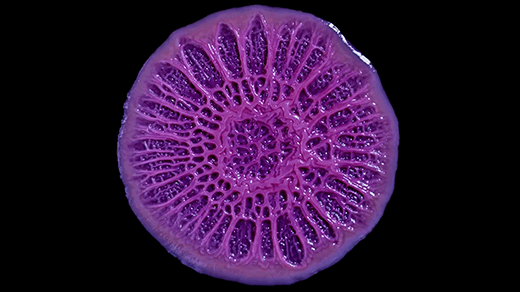
In his push to make these model systems truer to life, he has made seemingly fantastic scientific innovations. In his Palo Alto laboratory, Paşca has created miniature spherical tissues, or organoids, that resemble various regions of the human brain. He and his colleagues have linked organoids of brain, spinal cord and muscle tissue into “assembloids” that can twitch on command.
And in new work just announced today, Paşca’s team has shown that human organoids introduced into the developing brain of a young rat can spontaneously spread out and integrate themselves into the animal’s neural circuitry — a result that points the way toward research models of the human brain that are ever more lifelike yet practical and ethical to work on.
When the Vilcek Foundation of New York awarded Paşca their 2018 Prize for Creative Promise in Biomedical Science, they did so because his “effort led to a repository of patient-derived brain cultures that are among the most realistic mockups of brain development available to researchers today.”
According to his postdoctoral adviser, Ricardo Dolmetsch, the president of research and development at the gene therapy company uniQure: “Sergiu’s work raises the possibility that one day we will be able to transplant missing brain cells into people with disease or develop laboratory models of neurological or psychiatric diseases that we can use to develop medicines.”
We spoke with Paşca this past summer via Zoom and telephone. The interviews have been condensed and edited for clarity.
Did you always want to do scientific research?
From an early age. Yes.
I grew up in Romania, in a small city in Transylvania. As a child, I built a laboratory in the basement of my family’s house. I’d try to improve the growth of plants by adding different chemicals to the soil and then measuring their effect. Once, I added a copper-based molecule. It caused one of my plants to increase by 20%. That hooked me on research.
And today you grow nerve cells in a laboratory. How did that come about?
[Laughs.] It’s a long story. I attended school in the years after the overthrow of the Ceauşescu dictatorship. In that time, Romania was suffering from the long-term effects of dictatorship — isolation, underdevelopment. At the end of high school, I won a prize in a national contest in chemistry. The prize came with admission to any Romanian university. I picked Iuliu Haţieganu University of Medicine in Cluj-Napoca. The idea was to become a physician-researcher. I thought then, and still feel, that the world needs more of them.

Early in his career, Paşca’s dealings with the parents of children with neurological disorders affected him. “Talking with them was heart-wrenching,” he said. “It opened my eyes to the immense suffering the families went through.”
Rachel Bujalski for Quanta Magazine
Unfortunately, once at medical school, I discovered that there were few resources: no grants, no reagents for lab work. But I had one very dedicated professor, and she used 200 euros of her own money — a fortune at the time — to order a small reagent kit from Germany. We then planned for a year how to best use it.
And that’s how studying brain disorders first came up for me. I’d been considering using that reagent kit to test for metabolites in the blood of patients with cardiovascular disease. But to learn anything, I’d need to test hundreds, maybe thousands of patients. We had only enough reagents for 50 reactions!
One day, while in a statistics class, it hit me: The only way to do a study with a small cohort of patients would be to look at a disease that was rare. I thought: autism.
Autism? It’s not that rare — one in 50 have some form of it.
We didn’t know that 20 years ago.
My idea was to see if we could find signatures of the condition in the blood of children with autism. To do the study, I needed to convince parents to donate small amounts of their children’s blood. Talking with them was heart-wrenching. It opened my eyes to the immense suffering the families went through. The parents wondered, “What caused this?”
All I could say was, “Nothing is known.”
To be able to offer better answers, I signed up for a course in Bucharest offered by the International Brain Research Organization, the IBRO. They were American and British neuroscientists trying to bring advanced brain science to isolated countries. The clarity of their presentations and the beauty of the neuroscientific discoveries they described excited me immensely.
At the classes, I got to know Jack McMahan, one of the founders of the neurobiology program at Stanford. We stayed in touch, and later he helped me come to California.
What became of your metabolite study?
We discovered that some patients with autism had abnormalities in their one-carbon metabolism. This pathway, which is dependent on folate and B vitamins, was slightly disturbed, and this was likely related to a combination of genetic and nutritional factors.
By the time I finished medical school, I’d published several papers on autism. Jack McMahan read them and said, “Why don’t you come to Stanford? I have a colleague interested in moving his lab in this direction.” That was Ricardo Dolmetsch, who a few years later went on to become the global head of neuroscience at the Novartis Institutes for Biomedical Research.
It took a while to get funding, but eventually I received an IBRO fellowship and came to Palo Alto.
What was your assignment in the Dolmetsch laboratory?
To create a new approach for learning about the human brain.
A couple of years earlier, Shinya Yamanaka of the University of California, San Francisco and Kyoto University had figured out how to take skin cells from mice and reprogram them to turn back into induced pluripotent stem cells — iPS cells. Stem cells can be coaxed into all kinds of different cells, including neurons, the building blocks of the nervous system. Yamanaka would get the Nobel Prize for this.
In Ricardo’s lab, I planned to find ways to transform human iPS cells into neurons. The idea was to obtain skin cells from children with autism, turn them back into stem cells and then guide them to become neurons in a petri dish. If we succeeded, we hoped to leapfrog the barriers that have prevented us from fully understanding how the human nervous system develops. This would be a way to understand with greater clarity the biological basis of neuropsychiatric conditions like autism, epilepsy and schizophrenia.
What are these barriers?
The main problem is the human brain’s unbearable inaccessibility.
When something goes wrong in the spleen or the liver, doctors take a biopsy and analyze the tissue. This practice revolutionized medicine. Researchers have been able to take the cells of patients, put them in a dish, identify the malfunctioning mechanisms and apply different compounds to restore them. That’s how they’ve discovered new drugs.
But except in rare situations, we don’t drill through the skull of a living person to directly study human brain tissue. In addition to the medical risks, there are deep cultural taboos. We tend to associate the brain with “us,” with who we are. By touching the brain directly, these methods are in a way seen as interfering with the “self.”
Thinking back to my clinical rotations in medical school, I almost felt envious of my colleagues on the oncology ward. The molecular biology revolution, combined with the accessibility of the cancer tissues they were interested in, meant that they had new treatments in the pipeline. There were amazing things happening with leukemia, for example.
With autism, we had nothing. We couldn’t identify the mechanisms that caused the problems because we couldn’t directly study brain tissue. And even if we could, we wouldn’t have known what to look for.
Couldn’t you study human brain tissue obtained from autopsies?
The post-mortem brain tells you little about the electrical activity of living neurons. You need to measure that activity because that’s what neurons in the brain do: They fire electrical signals.
As for animal models, they have limitations when it comes to studies of psychiatric disorders. The human brain is more complicated than that of mice or even macaques. Millions of years of evolution separate us from these animals. We’ve seen countless examples of drugs that were highly successful in rodents and then failed in clinical trials in humans.
I thought we might move things forward by making some live human brain tissue ourselves.

Paşca sees the use of neural organoids as a way around the inherent difficulties of studying our living brains directly. “The main problem is the human brain’s unbearable inaccessibility,” he said.
Rachel Bujalski for Quanta Magazine
Your idea must have been controversial.
Oh, yes. There were people who thought it was not going to work. They thought that in transforming the skin cells, you’d lose the disease pathophysiology, and then we wouldn’t be able to discover anything new.
Yet within eight months, we had functioning human neurons from stem cells that had started out as the skin of patients with a genetic form of autism. Looking at them under the microscope, you could see them sparking calcium signals. We were getting somewhere.
How does one go about making neurons?
Cellular reprogramming got us stem cells from the skin cells. We then coaxed the stem cells to differentiate into other types of cells.
Stem cells love to differentiate. They need to turn into other cell types. And they actually have a high probability of becoming neurons almost by default. You don’t have to do that much to make neurons, though guiding them helps. What you do is, you spike the medium that the stem cells are kept in with molecules that promote transformation. Sometimes you take away some molecules, too.
We soon had millions of beautiful neurons. The bad news was that our neurons stuck to the bottom of a petri dish in a single cell layer, where after weeks in culture, they gave out. If we were to uncover what happens to the human brain as it develops over months upon months, we needed longer-lasting neurons.
I had an idea. I purchased a set of plastic laboratory dishes coated with a nonstick substance, and we grew the cells in those. Amazingly, the gambit worked! The cells couldn’t stick to the dish. Instead, they floated around in the medium maintaining them and aggregated into small balls the size of peas.
At first, we called these floating clumps of cells spheroids. Later they became known as organoids — something that resembled a specific organ but wasn’t one.
How were the cells in these balls different from your lone neurons?
They were growing in three-dimensional space. They moved around and interacted with one another. Importantly, they could be maintained in a dish for longer periods of time.
Under the right circumstances, we could keep the organoids for 900 days. And that permitted us to observe new things. For instance, at about nine or 10 months, the cells became more like postnatal neurons than prenatal ones. They seemed to have a sense of the passage of time, and what that should mean for their development.
How useful were the organoids for your research?
Let me tell you about an experiment we conducted with them.
There’s a genetic disease called 22q11.2-deletion syndrome that involves a loss of a part of the 22nd chromosome. Patients have a 30-fold increase in the risk of schizophrenia. They can also develop autism or other neuropsychiatric disorders. We recruited 15 patients and 15 healthy controls and started making neurons resembling the cerebral cortex from the skin they donated. We saw that the neurons from the patients had abnormal electric properties. They could not communicate with each other properly.
Now, schizophrenia is often treated with antipsychotic drugs. We put some of those drugs into a dish with cortical organoids made from our patients’ cells, and we saw that the antipsychotic drugs reversed the problem with the electrical properties of the neurons.
This meant we now had a way of testing these drugs in a dish.
You talked about organoids. But what are assembloids?
Assembloids are a new model system we came up with six to seven years ago. They are three-dimensional cell culture systems built from at least two different types of organoids, or by combining organoids with some other specialized cell types. By putting them together, we can see novel cell properties arising from their close interactions.
You can put two organoids resembling different brain regions together and see how the neurons project to one another and then connect to form circuits. Or you can combine organoids with immune cells and look at the neuroimmune interactions in disease.
For example, there’s a rare type of autism associated with a genetic disorder called Timothy syndrome. It is caused by a single-letter mutation in a gene encoding a calcium channel. It allows too much calcium to enter into cells when they receive electric signals. That interferes with the transmission of chemical signals within neurons and other excitable cells.
We took skin cells from patients with Timothy syndrome, made assembloids and then observed what happened within them. We could see that the neurons grown from the patients’ cells moved more often, but they moved for shorter distances than neurons from the healthy control group. The patients’ cells ultimately fell behind in their organization.
It must have been thrilling to witness this in real time.
You could see it! You could literally see it with your own eyes!
We had this dye that colored the calcium. The moment the calcium got into the cells, you could see the colors going up and coming down, measuring how much calcium got inside the cell. There was more of it in the patients’ cells.

The tiny spheres of brain organoids float in a culture dish in Paşca’s laboratory.
Paşca Lab, Stanford University
We’ve spent six years figuring out precisely how that calcium channel causes defects in the movement of these specific types of neurons. The mutated channel in patients impacts two different molecular pathways in neurons. Importantly, we found that you need two different drugs to restore the activity. We are thinking we now have the foundation for what might in the future get us to a treatment.
We would have never been able to learn this without the assembloids because you need the cells to interact in three dimensions to capture it.
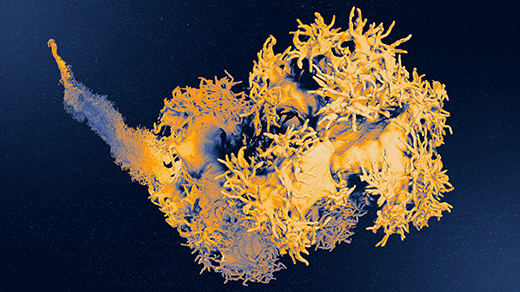
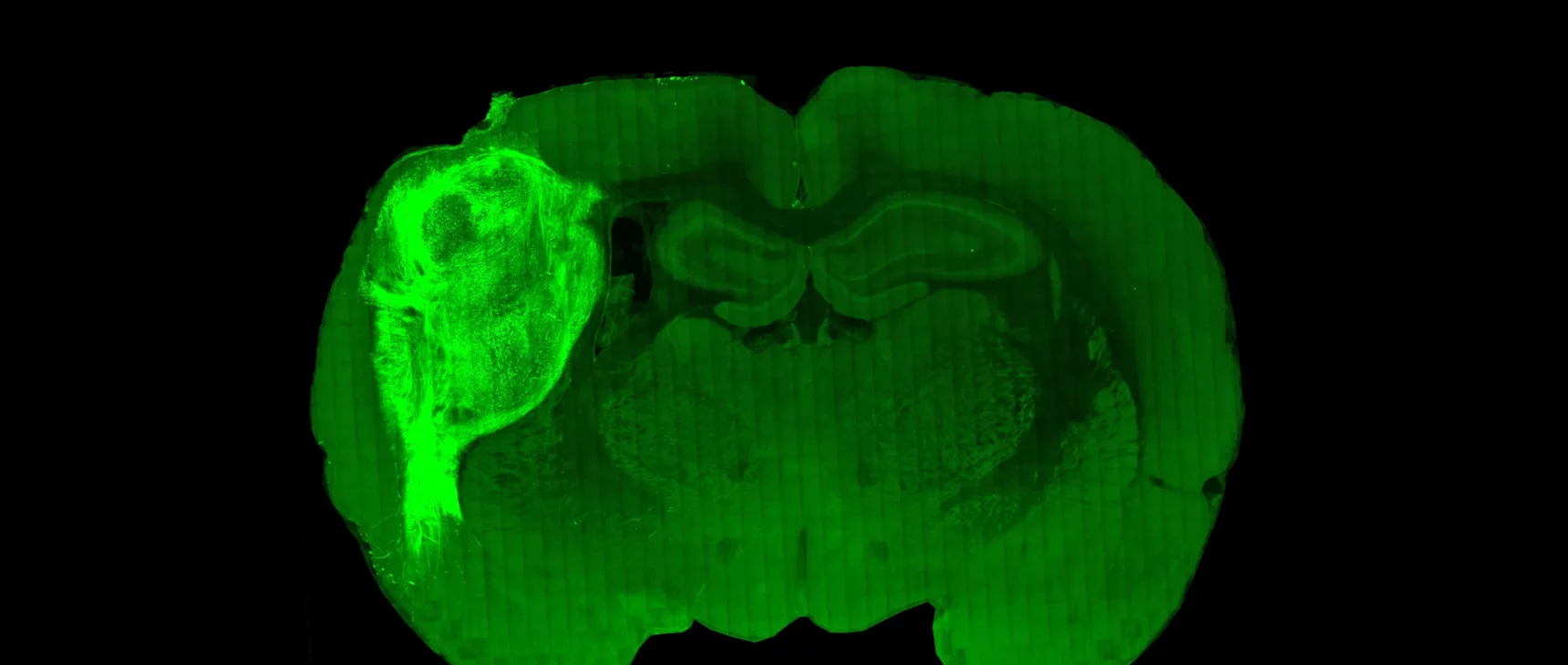
In your new paper, you announce that your lab created a rat in which human neurons cover one-third of the animal’s cortex in one hemisphere and deeply integrate into the brain. Why create this model?
For more than a decade, we’ve been making the cultures in a dish that recapitulate many aspects of a human’s nervous system. But there are limitations to these cultures: The neurons we’ve made don’t grow as large in size. There are no behavioral outputs as there would be in an actual human brain. And they don’t receive sensory inputs that would shape their development — the cortex needs to receive signals. We’ve been trying to provide some meaningful external input to these human neurons.
So the next step was growing human neurons inside the brain of a rat. We took the organoids and transplanted them into the cerebral cortex of a rat pup. The organoid became vascularized by the rat and eventually grew to cover one-third of its cerebral cortical hemisphere.
I thought you weren’t much of a fan of animal models for human brain research. What happened?
I think animal models and human cellular models are complementary. In this case, transplantation into animals allows us to integrate human neurons into circuits for understanding these diseases and to test drugs. It’s also another way of understanding how human neurons are processing information within living circuits.
So these human neurons from the organoids have had a chance to grow within rat brains, and they have been able to get inputs and outputs from the animal. How do they compare to neurons that have only grown within the organoids? And how do they compare to the neurons growing in our own brains?
Transplanted human neurons are about six times larger than human neurons of comparable development maintained in a dish. They also are more mature electrophysiologically and form more synapses and are much closer to neurons in the postnatal human brain.

“Transplantation into animals allows us to integrate human neurons into circuits for understanding these diseases and to test drugs,” Paşca said. “It’s also another way of understanding how human neurons are processing information within living circuits.”
Rachel Bujalski for Quanta Magazine
Do you see any kinds of behavioral differences in these rats that have acquired so many human neurons?
No. There are no differences in the cognitive and motor tasks in which we tested the rats. We also verified and they don’t experience seizures. The rats can, however, be trained to associate stimulation of human neurons with delivery of a reward. This offers unprecedented opportunities to study human brain disorders.
At what point should your assembloids and organoids have some kind of legal rights?
I think for in vitro cultures, they are just clusters of cells. We don’t consider them brains. It gets more nuanced when it comes to transplanting into animals.
How do bioethicists feel about your experiments?
We work closely with them. All along this path, I’ve been actively involved in discussions with ethicists at Stanford and beyond. All the experiments we do are closely monitored and discussed with ethicists. We don’t do the experiments in isolation. Experiments are discussed before they are undertaken and certainly while they are running. We’ll talk about the implications, the pros and cons.
Do you ever think about the Frankenstein story?
I think a lot about it. But I think the story isn’t all that relevant to today’s science. In today’s world, technologies can be developed that are ethical. A lot has to do with the motive of the researcher. My long-term goal is to find a treatment and perhaps a cure for these neurodevelopmental disorders. That’s been my North Star.
Some years ago before she received the Nobel Prize for CRISPR, I asked Jennifer Doudna if she was worried about the potential misuse of this gene editing technology. She replied that she was concerned about “the desire in some areas to rush forward to change human embryos.” Not long afterward, an ambitious researcher in Shenzhen, China, announced he’d used CRISPR to revise the genetic code of two human babies. Do you ever worry about picking up the newspaper and discovering that a scientist somewhere has used your work to prematurely produce parts of a human brain?
No. One of the things that distinguishes organoid work from CRISPR is the resources needed for the experiments. To do a gene edit with CRISPR requires only some training and few resources. It’s possible to do it in your kitchen! What we do requires a lot more time and money. To keep cells alive for 900 days is quite expensive and requires specialized training and facilities. That fact alone gives us some breathing room to process our discoveries and their implications.
There are few places with the infrastructure and expertise needed to do this. We’re trying to replicate human brain development, which takes a very long time. Uncovering its hidden engineering takes even longer. I’ve been working at this every day for the last 15 years.
How close are you to some solutions?
I’m hopeful, but I don’t want to be unrealistic. We’re certainly better off than we were 15 years ago. We now have long lists of genes associated with autism and we have this new tool to study them. But we still need to understand how those mutated genes cause things to go wrong in the brain so that we can build effective drugs.
Your story begins in Romania 20 years ago when you didn’t know what to say to the parents of children with autism. If you went back to that country now, what would you say?
All I could honestly say is that I’m hopeful. We’re still far away from having a cure. On the other hand, there have been huge breakthroughs in other seemingly insoluble diseases in recent years. That gives me tremendous hope.