Chemists Seek Possible Precursor to RNA
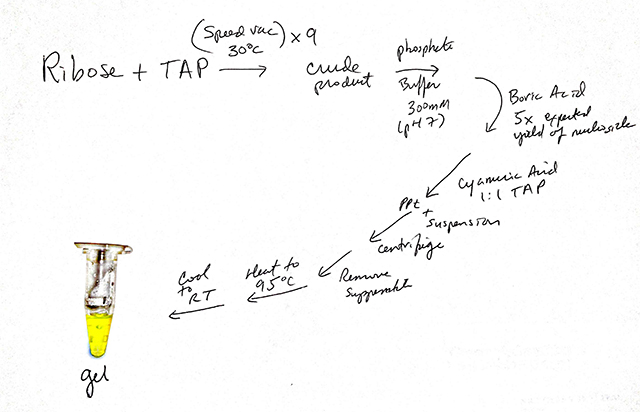
For Nicholas Hud, a chemist at the Georgia Institute of Technology, the turning point came in July of 2012 when two of his students rushed into his office with a tiny tube of gel. The contents, which looked like a blob of lemon Jell-O, represented the fruits of a 20-year effort to construct something that looked like life from the cacophony of chemicals that were available on the early Earth.
To some biochemists, Hud’s attempts to find an evolutionary precursor to ribonucleic acid may have seemed a fool’s errand. The dominant theory to explain the origins of life — known as the RNA world hypothesis — regards ribonucleic acid as the first biological molecule. Its allure comes from the molecule’s dual nature. Unlike DNA, the molecule that provides the blueprint for all living things, RNA acts as both an information carrier and an enzyme, catalyzing reactions. That means the molecule has the potential to copy itself and to pass along its genetic code, two essential components for Darwinian evolution.
If RNA was indeed the first biological molecule, discovering how it first formed would illuminate the birth of life. The basic building blocks of RNA were available on prebiotic Earth, but chemists, including Hud, have spent years trying to assemble them into an RNA molecule with little success. About 15 years ago, Hud grew frustrated with that search and decided to explore an alternative idea: Perhaps the first biological molecule was not RNA, but a precursor that possessed similar characteristics and could more easily assemble itself from prebiotic ingredients. Perhaps RNA evolved from this more ancient molecule, just as DNA evolved from RNA.
Hud’s team started exploring this idea explicitly a decade ago. When the gel formed in 2012, after the testing of dozens of chemicals, Hud’s team knew it had made a significant advance in the chemistry of a possible proto-RNA world. After years of failed attempts, a surprisingly simple chemical recipe had produced a conglomerate of long, ribbonlike molecules whose structure and chemical components resembled those of RNA.
Hud immediately asked the students to recite the protocol they had used for the reaction, scribbling it down as they spoke. “I wanted to be sure that we would always remember how they had obtained [the end-product] by such a simple procedure,” he said. In December 2013, the results were published in the Journal of the American Chemical Society.
“In my opinion, nothing like this has been seen before,” said Stephen Freeland, a biologist at the University of Maryland Baltimore County, who was not involved in the study. Although he isn’t certain that the chemicals Hud picked will end up being the precise components of proto-RNA, Freeland said Hud has “made conceptual progress.”
Hud isn’t the first scientist to explore an alternative chemistry for RNA. But the robustness of his reaction is unique — the molecules seem to seek one another out, reacting without a lot of chemical coaxing. Hud and others say this ease of creation is essential for reactions to have taken place in the chaotic chemical cauldron of early Earth. “Before this, people just didn’t focus on the real-world situation,” Freeland said. “We need something so robust that no matter what the situation is, it will still happen.”
Hud’s team is now testing whether its reactions will work in a messy mix of molecules more analogous to the primordial soup.
Hud’s chemistry — and the concept of proto-RNA in general — still faces hurdles. His molecule possesses a polymer-like structure of repeating units similar to nucleic acids. In RNA and DNA, the sequence of those units is essential for carrying information, allowing those molecules to store and transmit the code of life. But Hud’s molecule uses only two chemical letters, compared with RNA’s four, and the repeating units can easily come apart. That means it doesn’t have the informational content of RNA, an essential characteristic of life.
Proponents of the traditional RNA world hypothesis say that moving from an RNA precursor like Hud’s to RNA itself still represents an incredible challenge, possibly as daunting as making RNA from scratch. If these molecules were successful enough to launch the origins of life, where are they now?
“To me, the proto-RNA idea raises more questions than it answers,” said John Sutherland, a chemist at the MRC Laboratory of Molecular Biology in Cambridge, England, who nonetheless described Hud’s work as elegant and well done. “If it’s too difficult for RNA to assemble chemically, how can a primitive biology invent RNA?”
From Soup to Structure
In the modern cell, cooking up an RNA molecule is a complex process involving multiple enzymes that link a sugar (ribose) to one of four nucleobases — chemical letters that make up the genetic code and come in the flavors guanine, adenine, uracil and cytosine — and a phosphate, which provides the backbone of the structure. Another enzyme ties together repeating units of each of these three components into the long chain of RNA.
But in the pre-biotic Earth, there were no enzymes. So how could the first RNA molecules have formed? According to the RNA world hypothesis, RNA spontaneously came together through geochemical processes. Scientists studying the origins of life have spent the past 40 years trying to figure out exactly how this could have happened, analyzing the likely chemical components of early Earth and devising chemical reactions to bring them together. “The chemistry of making RNA is so difficult that it’s hard to imagine that you could have a one-pot reaction, where molecules come together and spontaneously make this complex molecule,” Hud said.
Scientists have been able to produce a few of these components without enzymes. In 2009, Sutherland and collaborators showed for the first time that they could synthesize one of the basic units of RNA from scratch. They argue RNA could have formed this way in nature, but Hud and Freeland say the precise chemical conditions and steps required for the reaction would have been unlikely to occur in the chaotic chemical cauldron of prebiotic Earth.
An alternative hypothesis is that RNA as we know it has undergone substantial chemical and biological evolution. “The origins of life and the origin of the genetic code are no longer synonymous,” said Antonio Lazcano, a biologist at the National Autonomous University of Mexico in Mexico City and former president of the International Society for the Study of the Origin of Life who was not involved in Hud’s study. “You can have a significant part of the genetic code that will be the outcome of biological evolution and a largely undescribed stage of chemical evolution.”
My Grandfather’s Ax
Scientists have long considered alternative chemistries for RNA, synthesizing molecules with alien components that have even found their way into biotechnology applications. Nicholas Hud, a chemist at the Georgia Institute of Technology, takes a broader approach — perhaps every component was different and each changed over time. To explain, Hud employs an ancient Greek paradox called “my grandfather’s ax”: If your father replaced the handle and you replaced the blade, the result would be an entirely new ax. “Everyone accepts that DNA comes from RNA and DNA is harder to make than RNA,” Hud said. “So if you’re willing to accept that DNA evolved from RNA, then why not that RNA is product of evolution of proto-RNA?”
Scientists have been examining molecules with alternative bases or sugars almost since RNA was proposed as the first biological molecule in the 1960s. But this approach creates an overwhelming set of possible permutations, as each of the three components — sugar, phosphate and base — has numerous potential replacements. “The chemical space becomes enormous,” Hud said. “It’s a really big task to find out what came first.”
Hud’s team started with the bases, looking for candidates that could form something like the traditional base pairs of RNA and DNA, in which certain bases seek each other out like lost lovers; in RNA, adenine binds only with uracil and guanine with cytosine. It’s this pairing that enables the molecules’ unique capacity to store information. Each molecule acts as a template for the next generation, creating a sort of mirror image of its predecessor.
But Hud also wanted base pairs that, unlike traditional bases, could spontaneously assemble into long polymers. “If you have a complex mixture of thousands of molecules, the chemistry relies on what reacts the fastest,” Hud said. “The molecules need to organize themselves.”
Rather than limit themselves to the four bases used in RNA, the members of Hud’s team considered a library of roughly 100 structurally similar molecules, including only those that were predicted to have existed on prebiotic Earth or in meteorites, which may have carried with them essential components of life. “We’re foolish if we don’t think about this: either why nature picked these four or what nature did before picking these four,” Freeland said.
Molecular Recipes
To try to find bases that bond like those of RNA, Hud’s team started mixing chemicals under various conditions. After several years, the researchers homed in on a few promising candidates, most notably two molecules, triaminopyrimidine (TAP) and cyanuric acid (CA). Last year, in a paper published in the Journal of the American Chemical Society, the researchers showed that a slightly modified version of triaminopyrimidine and cyanuric acid self-assemble in water, creating something that resembles traditional base pairs. However, rather than the conventional duo of base pairs, adenine and uracil or cytosine and guanine, the molecules form hexamers, or six-membered rings. The hexamers stack on top of one another, forming long, polymerlike structures. They had found a chemical pairing that spontaneously assembled into a complex, RNA-like arrangement. “We were surprised it worked so well,” Hud said.
Hud’s team set out to tackle the next problem in RNA assembly: How do bases attach to the ribose sugar? In their newest paper, published in the same journal, the researchers showed that TAP and ribose easily bond when mixed in water, creating molecules known as nucleosides. (The finding was especially encouraging because this bond has been difficult to form between sugars and traditional RNA bases.) When the researchers added the other base, CA, and heated the mixture, it formed into long polymers, about the length of genes. It’s these polymers that create the gel that excited Hud’s team.
“I think it’s an important step because it shows that the physical forces that hold genomes together today can be reproduced in the protoworld,” said Frank Schmidt, a biochemist at the University of Missouri in Columbia who was not involved in the studies. “He has shown that you can start with star stuff [chemicals originally produced by stars] and get something with some of the fundamental properties of RNA.”
The beauty of Hud’s chemistry is that the assembly doesn’t require an enzyme or a template — the molecules come together on their own.
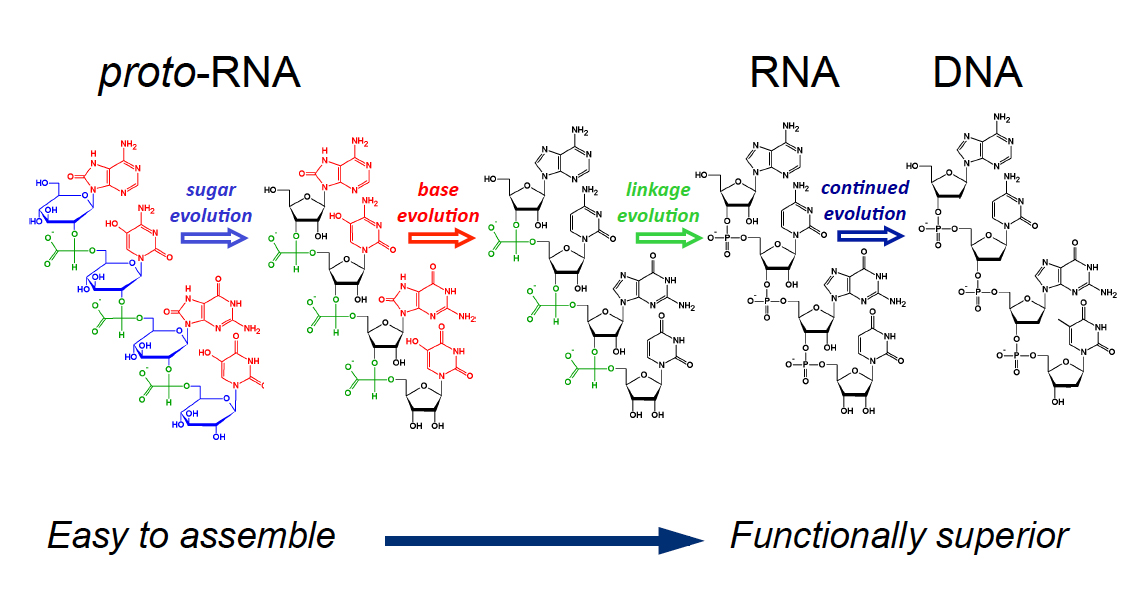
However, there are still important differences between Hud’s polymer and RNA. “These lovely properties come at the price of taking a step away from the chemistry we all know,” said Michael Yarus, a molecular biologist at the University of Colorado in Boulder who was not involved in the studies. For example, unlike RNA, each molecule in the stack is linked by a relatively weak kind of bond known as a non-covalent bond. Like a set of magnetic beads that can break apart and reconnect, the structure can separate more easily than RNA, which is more similar to beads knotted on a string. That flexible structure impairs the polymer’s potential to reliably store information in the sequence of bases, which makes up the code of life.
Other big questions include why and how these molecules could have evolved into modern RNAs, considering that it might have been easier for the precursor molecule to maintain the status quo. Proponents of the traditional RNA world view this as a giant obstacle, but Hud disagrees. CA can be converted into uracil and TAP into guanine and adenine with only a few chemical changes, he said. His team is now exploring other candidate bases capable of forming pairs and self-assembling with ribose sugars. The researchers are also looking at alternatives for the other components of RNA, the sugars and phospates, as well as how to stitch together nucleosides in a way that mimics the knotted string of RNA. Even though the final result may look quite different than RNA, Hud argues that because RNA is the superior system, natural selection will favor its creation and drive its precursor to extinction.
Even those who are not convinced of the proto-RNA world say it’s worth exploring the possibilities. “It’s important to have a lot of routes to find the one that really happened, the one that’s highly probable,” Yarus said, adding that how far Hud’s chemistry will travel along that path of probability is not yet clear.
Others are looking at an even broader set of chemical alternatives. In a paper published in November 2013, Freeland and collaborator Jim Cleaves, a chemist at the Earth-Life Science Institute in Tokyo, used computational methods to examine alternative amino acids, which are the building blocks of proteins. The team plans to do the same for the building blocks of RNA. “Hud’s list is just the tip of the iceberg,” Freeland said. “There could be tens of thousands of structures to seriously consider.”
This article was reprinted on Wired.com.