Killer Virus Is Invading Koala DNA
In the 1990s, Jon Hanger was working as a part-time veterinarian at Dreamworld, a theme park in Queensland, Australia. Koalas are revered in Australia, where their natural habitat ranges across the country’s east. But the koalas of Dreamworld were dying. A large number of them were succumbing to cancer, particularly leukemia and lymphoma.
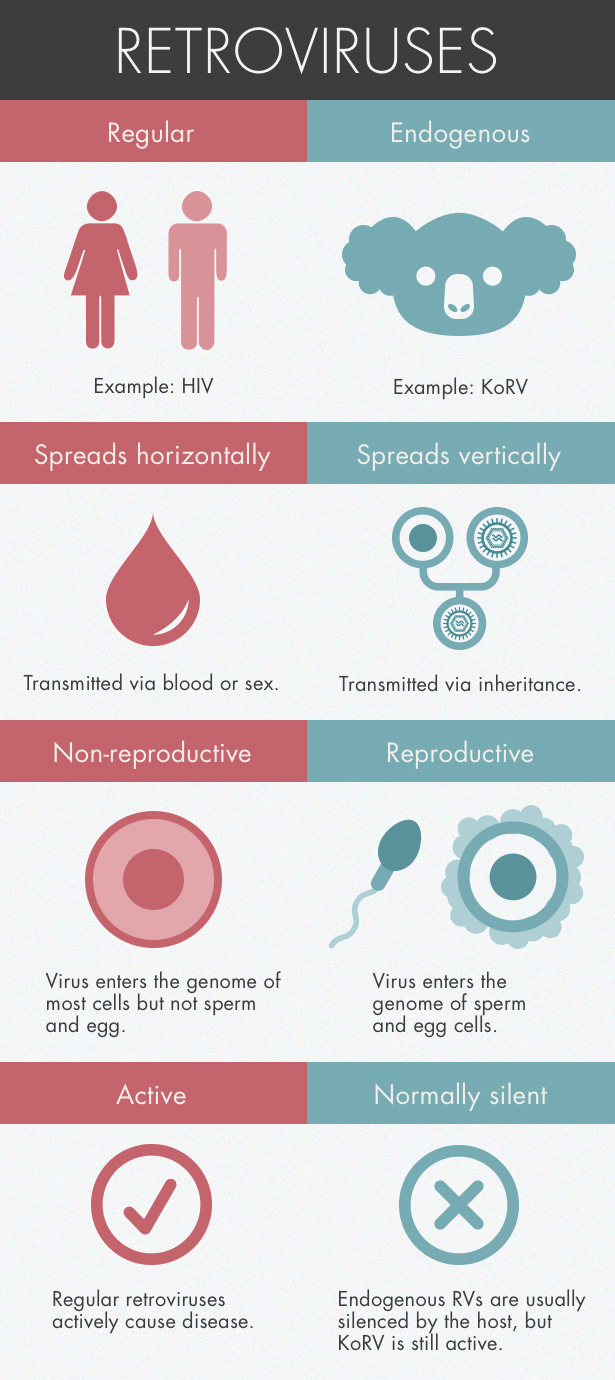
In the veterinary realm, cancer clusters are often the sign of a retrovirus, a type of virus that inserts itself into its hosts’ genome. Hanger and his colleagues tested a total of 200 animals, both captive and wild, and found that every single one showed signs of a novel retrovirus. The shocking pervasiveness suggested that rather than being transmitted from animal to animal like the flu, the virus had wormed its way into the koalas’ sperm and egg cells and was being passed down from parent to child just like any other gene.
Such a retroviral jump into the germ-line genome isn’t unusual on evolutionary timescales. All vertebrates harbor retroviral DNA, molecular relics of infections from the distant past. Human genomes are made of about 8 percent retrovirus DNA.
But in humans and most other animals, the viral DNA entered our genomes so long ago that it no longer makes us sick. In koalas, however, the viral integration seems to have occurred very recently, and is perhaps even ongoing — an unprecedented event in virology. For the first time, scientists have the opportunity to track the process in its earliest stages.
Scientists want to understand how retroviruses integrate into the genome of their hosts because it can have a profound effect on the hosts’ evolution. Viral DNA can protect animals against further infection — a kind of integrated viral defense system. The new retroviral DNA can even provide new genes. Indeed, such DNA helped the placenta form, which in turn led to the rise of mammals.
But because most retroviral infections happened so long ago, researchers know relatively little about how they became a permanent part of us. “We don’t know the factors that allow these sequences to get into the genome in the first place and become part of the gene pool,” said Welkin Johnson, a virologist at Boston College. Virologists are looking for answers in the sick koalas of Queensland.
Sickness Spreading
It’s no accident that the koala retrovirus (KoRV) was discovered in a theme park. In the many parts of the country where the koala population has suffered from disease and habitat loss, they are protected, making koala diseases particularly difficult to study. Scientists can’t treat koalas as they would lab mice, intentionally infecting them with KoRV to directly investigate its impact. Much of the research on KoRV has taken place on dead or injured koalas that have been brought into veterinary clinics after being hit by cars or attacked by dogs.
And then there are the claws. “No vet would handle a wild koala that wasn’t sedated,” said Rachael Tarlinton, a veterinarian at the University of Nottingham who conducted much of the early research on KoRV as a graduate student at the University of Queensland. She tells the story of how a driver once found an unconscious koala, put it into the trunk of the car, and drove it to the hospital. Upon arrival, Tarlinton had to carefully extract the animal from the vehicle, as it had awoken en route and embedded its claws in the trunk.
Tarlinton first noticed that KoRV was unusual when she looked for it in wild koalas in the south. Tarlinton knew that nearly 100 percent of animals tested in Queensland, the northeastern-most state in Australia, had KoRV. But Australia’s southern koalas have a very different history. In the late 19th and early 20th centuries, southern populations were hunted almost to extinction for their furs. Early conservationists intent on reviving the species transplanted koalas to some of the country’s isolated southern islands, where they have thrived.
In an analysis published in 2006, Tarlinton and her colleagues tested 26 koalas on Kangaroo Island, which lies a few miles off the South Australian coast. None of them showed signs of the virus. Meanwhile, regions of Australia between Queensland and the southern islands had koala populations with moderate KoRV levels, suggesting that the virus is marching south.
The spreading sickness implies that the retrovirus is in the middle of a transition period, the type of which researchers have never been able to study in the wild. When a retrovirus first infects a species, it’s transmitted from animal to animal. The retrovirus replicates within the host by integrating into the animal’s genome, making it impossible for the host to shake. (Sometimes that insertion can trigger a cancer-linked gene, explaining the link between retroviruses and cancer.) The virus becomes “endogenous” when it breaches the genome of germ cells — those cells that create eggs and sperm — transforming the virus into a permanent part of the gene pool. Because germ cells can form embryos, the virus will be passed to the offspring just like any other gene.
Researchers have found KoRV DNA in koala sperm, confirming that the virus has indeed taken root in the germ line. But it’s also circulating in the bloodstream, which is unusual for endogenous viruses. The combination indicates that KoRV is still spreading from animal to animal, said Paul Young, a virologist at the University of Queensland who has been studying KoRV for more than a decade. “We had never seen this before.”
Because koalas are in this transition period, researchers hope to illuminate one of the great mysteries about the spread of retroviruses: How does a deadly virus become one with the host without killing off the host population entirely? “If you can answer that, you can explain 10 percent of the vertebrate genomes,” said Alex Greenwood, an evolutionary virologist at the Leibniz Institute for Zoo and Wildlife Research in Berlin.
Greenwood’s team has compared virus and koala sequences from different populations in the north and south, which have had to grapple with the virus for different lengths of time. With this information they hope to reveal the changes that occur in the virus in order for it to become part of the genome.
Of course, the retrovirus doesn’t enter the host’s genome just one time. Northern koalas have anywhere from 10 to 300 copies of the virus, suggesting that it entered the germ line repeatedly — perhaps tens of thousands of times over the course of hundreds or thousands of years. The initial infection could have come as long as 49,000 years ago, according to a genetic analysis published this year.
Greenwood’s team plans to analyze 10,000-year-old koala fossils, which they hope will tell them what kind of impact the initial infection had on the species. “Once we know how old KoRV really is, we can use museum specimens and fossils to see if there is evidence that koalas had a population crash that correlates with the first invasion,” he said.
Regardless of when that first infection happened, in the years since then KoRV seems to have lost some of its potency. Compared to its nearest relative, a retrovirus that infects gibbons, KoRV has acquired two mutations that lower its virulence. If KoRV follows the path of other endogenous retroviruses, that trend will continue. “We think it will gradually lose its ability to hurt the host,” said Alfred Roca, a geneticist at the University of Illinois, Urbana-Champaign.
The Long View
Over the course of millions of years, changes in a retrovirus can even bring benefits to the host. Indeed, one reason scientists are so interested in retroviruses is their potential to drive the development of new species. “People talk about how the genomes of chimps and humans are 96 percent identical, but the retroviral part of our genome is much more different,” Greenwood said. “That part of the genome is very interesting, and trying to understand it is critical.”
When a retrovirus permanently settles into the genome, it can have broad, unpredictable effects. Viruses carry machinery to activate genes, which can work on both viral and host DNA. And cells have mechanisms to silence viral genes, which can inadvertently turn off the hosts’ genes as well. Occasionally, viral components trigger host genes that spur uncontrolled cell growth, otherwise known as cancer. “A lot of cancers come about because the viruses themselves are powerful replicating machines,” Young said.
Around 40 million years ago, our primate ancestors suffered a retroviral epidemic that had an enormous impact on their descendants. As the virus integrated into the genome of its primate hosts, most of its genes went silent. But one gene remained — a gene that viruses use to gain entry into their target cell. Our mammalian ancestors coopted that gene, now known as syncytin, for a new purpose: to help form the placenta, the connection between mother and fetus. Remarkably, a similar event occurred multiple times independently in mammalian evolution, with different animals appropriating syncytins from different retroviruses.
“Endogenous retroviruses are such a huge component of genomes that understanding where they came from is understanding how vertebrates evolved,” Johnson said. “In some sense I think it’s an underappreciated source of genetic variability.”
Such potential benefits for koalas remain in the far future, however. While some koala communities are so abundant that they are endangering certain species of eucalyptus, their main food source, others are suffering as human development encroaches on their territory. For the koalas of Australia, KoRV is making a difficult future worse.
Editor’s note: This article originally appeared with the headline “Killer Epidemic Is Invading Koala DNA.” We altered the headline on March 6 to reflect the fact that an “epidemic” is an outbreak of disease, and so can not itself invade genomes.