Lab-Grown Human Cells Form Working Circuits in Rat Brains
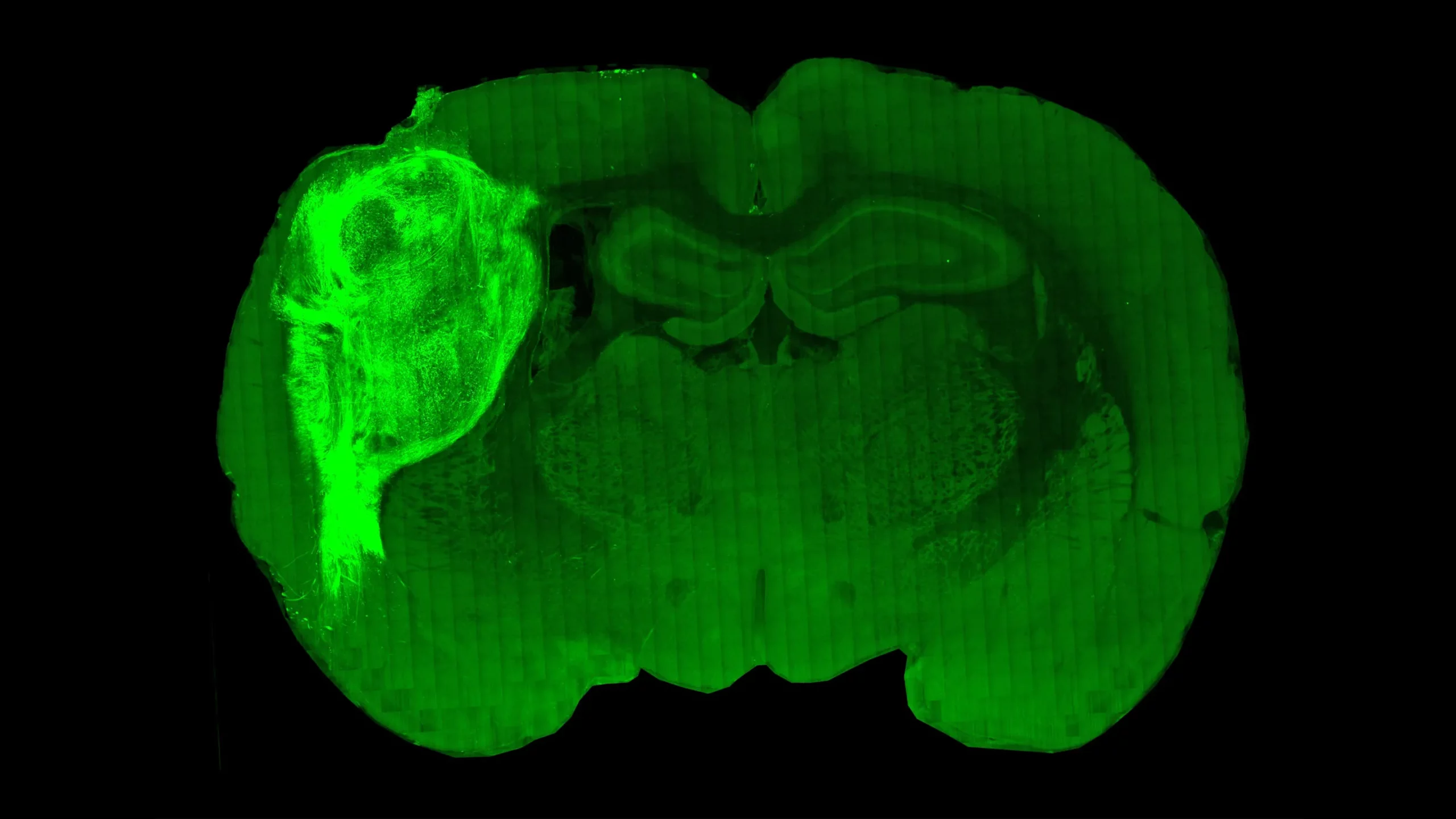
A cross section through the brain of a laboratory rat reveals the extensive growth of a human brain organoid (bright area on left) transplanted into it. New work shows that the human tissue not only grows but forms working connections with neurons from the rat’s brain.
Paşca Lab, Stanford University
Introduction
Our understanding of the inner workings of the human brain has long been held back by the practical and ethical difficulty of observing human neurons develop, connect and interact. Today, in a new study published in Nature, neuroscientists at Stanford University led by Sergiu Paşca report that they have found a new way to study human neurons — by transplanting human brainlike tissue into rats that are just days old, when their brains have not yet fully formed. The researchers show that human neurons and other brain cells can grow and integrate themselves into the rat’s brain, becoming part of the functional neural circuitry that processes sensations and controls aspects of behaviors.
Using this technique, scientists should be able to create new living models for a wide range of neurodevelopmental disorders, including at least some forms of autism spectrum disorder. The models would be just as practical for neuroscientific lab studies as current animal models are but would be better stand-ins for human disorders because they would consist of real human cells in functional neural circuits. They could be ideal targets for modern neuroscience tools that are too invasive to use in real human brains.
“This approach is a step forward for the field and offers a new way to understand disorders of neuronal functioning,” said Madeline Lancaster, a neuroscientist at the MRC Laboratory of Molecular Biology in Cambridge, U.K., who was not involved in the work.
The work also marks an exciting new chapter in the use of neural organoids. Nearly 15 years ago, biologists discovered that human stem cells could self-organize and grow into small spheres that held different types of cells and resembled brain tissue. These organoids opened a new window into the activities of brain cells, but the view has its limits. While neurons in a dish can connect to each other and communicate electrically, they can’t form truly functional circuits or attain the full growth and computational prowess of healthy neurons in their natural habitat, the brain.
Pioneering work by various research groups proved years ago that human brain organoids could be inserted into the brains of adult rats and survive. But the new study shows for the first time that the burgeoning brain of a newborn rat will accept human neurons and allow them to mature, while also integrating them into local circuits capable of driving the rat’s behavior.
Paşca pointed out that there were “a thousand reasons to believe that would not work,” given the drastic differences in how and when the nervous systems of the two species develop. And yet it did work, with the human cells finding the clues they needed to make essential connections.
“This is a much needed and elegant study that steers the field in the right direction of looking for approaches to advance the physiological relevance of human brain organoids to model later stages of human brain development,” said Giorgia Quadrato, a neuroscientist at the University of Southern California.
Understanding the cellular and molecular processes that go awry in neurons and lead to brain disorders has always been Paşca’s motivation. [Editor’s note: See the accompanying interview with Paşca about his life, career and motivations for his work.] Because many psychiatric and neurological disorders take root in the brain during development — even though symptoms may not emerge until years later — watching how neurons develop has seemed like the best way to fill the voids in our understanding. That’s why it’s been Paşca’s goal to transplant human brain organoids into newborn rats ever since he began working with neurons in a dish 13 years ago.
In the new work — which was also headed by Paşca’s Stanford colleagues Felicity Gore, Kevin Kelley and Omer Revah (now at the Hebrew University of Jerusalem) — the team inserted cortical human brain organoids into the somatosensory cortex of very young rat pups, before the pups’ brain circuitry was fully established. This gave the human neurons a chance to receive long-range connections from a key region that processes incoming sensory information. Then the researchers waited to see whether the organoid would grow in concert with the rest of the rat’s developing brain.
“We discovered that if we put the organoid in at that early stage … it grows up to nine times larger than it initially was over a period of four or five months,” said Paşca. That translated to an area of humanlike brain tissue that covered about a third of one of the rat’s brain hemispheres.
But even though the human neurons stayed together in the cortical area where they were surgically placed, the researchers demonstrated that they became active parts of the neural circuitry threaded deep within the rat’s brain. Most of the transplanted human neurons began responding to touch sensations from the rat’s whiskers: When puffs of air were directed at the whiskers, the human neurons became more electrically active.
Even more surprising, the flow of neural signals could also run in the other direction and influence behavior. When the human neurons were stimulated with blue light (through a technique called optogenetics), it triggered a conditioned behavior in the rats that made them seek a reward by licking more often at a water bottle.
“That means that we have actually integrated human cells into the circuitry,” said Paşca. “It’s not changing the circuits. … It’s just that human cells are now part of it.”
The transplanted cells didn’t perfectly mimic human brain tissue in their new setting. For example, they did not organize themselves into the same multilayered structure seen in the human cortex. (Nor did they follow the lead of surrounding rat neurons and form the barrel-like columns characteristic of the rat somatosensory cortex.) But the individual transplanted neurons did keep many of the normal human electrical and structural properties.

Sergiu Paşca, professor of psychiatry and behavioral sciences at Stanford University and director of the Stanford Brain Organogenesis Program at the Wu Tsai Neurosciences Institute.
Rachel Bujalski for Quanta Magazine
The cells took advantage of one major perk of being inside a brain: They successfully linked up with the rat brain’s vascular system, allowing blood vessels to permeate the tissue to deliver oxygen and hormones. The lack of a blood supply is thought to be a major reason why human neurons growing in a dish routinely fail to mature fully, along with a lack of neural signal inputs that are probably needed to shape development, Paşca explained. When his team compared the transplanted human neurons to those living in a dish, they found that the transplanted neurons were six times larger, with a size and electrical activity profile closer to that of neurons from natural human brain tissue.
“There’s something about the in vivo environment — so, the nutrients and electrical signals that they’re receiving in the brain — that brings human cells to another level of maturation,” Paşca said.
Because the human neurons matured so much within the rat brains, Paşca and his colleagues could see unusual differences in the development of brain organoids derived from people with a genetic disorder called Timothy syndrome, which often causes autism and epilepsy. In the rat brains, the transplanted human neurons carrying genes for Timothy syndrome grew abnormal dendritic branches that made unusual connections. Crucially, some of these atypical developments could only be seen in human neurons growing within the rat cortex, and not in organoid neurons in a dish.
Paşca emphasizes that until now, these types of subtle changes in maturing neurons that affect brain function and lead to neurological and psychiatric disorders have been largely hidden from us.
“The results are very exciting,” said Bennett Novitch, a neuroscientist and stem cell biologist at the University of California, Los Angeles. In vitro studies of neural tissues will still be quicker and more practical for many types of neurological studies and drug tests, he noted, but the new paper “illustrates how revealing the mature characteristics of human neurons … is still best achieved in the in vivo setting.”
Paşca hopes that being able to study mature human neurons within rats will finally bring treatments for psychiatric disorders and neurological conditions closer. Others in the field are hopeful too. “If this organoid transplantation strategy can truly mimic disease signatures, this could really accelerate our path toward cures,” said Joel Blanchard, a neuroscientist at the Icahn School of Medicine at Mount Sinai.
The nature of the new work may raise questions about the welfare and ethical treatment of the rats. For that reason, Paşca and his colleagues have held active discussions with ethicists from the beginning. As in all experiments involving animals, there was a legal requirement that the rats be extensively monitored by lab technicians with the authority to stop the experiment at any time. But no differences were found in the rats with transplanted human brain organoids in an array of behavioral and cognitive tests.
Insoo Hyun, a bioethicist affiliated with Harvard Medical School’s Center for Bioethics, said he doesn’t have any ethical concerns about the current experiments. Paşca’s team followed all the guidelines developed by the International Society for Stem Cell Research governing research with human brain organoids and the transfer of human cells into animals. “To me, the issue is really understanding: Where do you go from there?” he said.
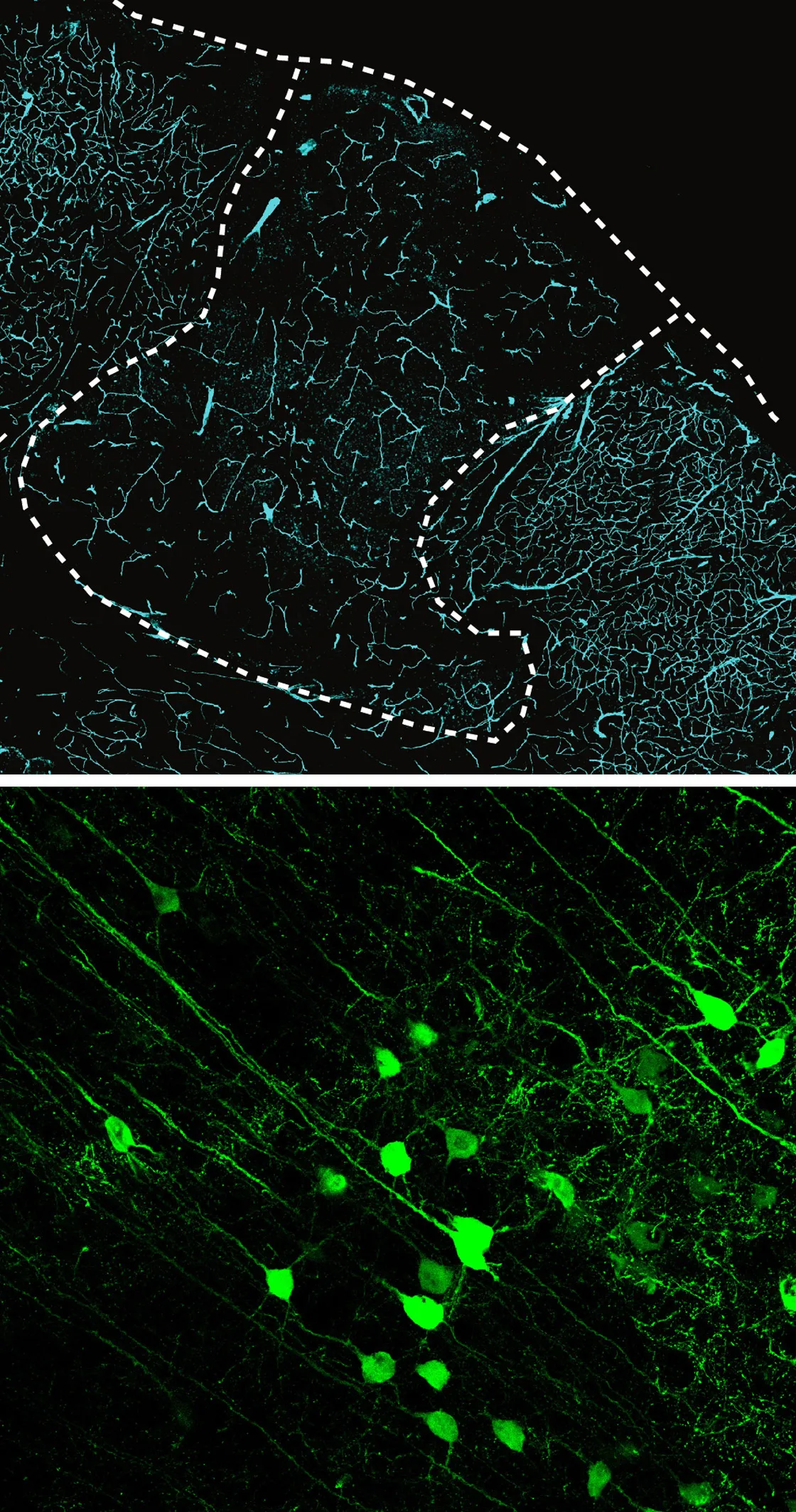
In a cross section through the cortex of one of Paşca’s experimental rats (top), the dotted line marks the edge of the transplanted human organoid. The staining shows that blood vessels from the animal have richly vascularized the human graft. In another experiment, the green glow in rat neurons (bottom) located far from the implanted organoid shows that they have formed working synapses with the human cells.
Paşca Lab, Stanford University
Hyun is more concerned about other research teams that may now become interested in transplanting human brain organoids into species more similar to our own, such as nonhuman primates. “You would have to have a very intense conversation at the oversight level of why you’re justified in going to something more complex,” Hyun said.
Paşca says that he and his colleagues have no interest in such boundary-pushing experiments. He also thinks that the difficulty of growing and sustaining organoids for transplantation will curb most potentially reckless research. “There are few places with the infrastructure and expertise needed to do this,” he said.
The more immediate and practical scientific challenges lie in improving the human brain organoids that get transplanted into rats. Unquestionably, there is still a long way to go. The human brainlike tissue is currently missing many important brain cells beyond neurons, like microglia and astrocytes, as well as neurons involved in inhibiting the activity of other neurons. Paşca’s team is currently working on experiments that will transplant “assembloids” — sets of organoids representing different brain regions whose cells migrate and interact with one another.
There may be limits to how much the findings from human neurons within a rat brain can apply to a natural human brain. The rats used in these transplant studies are born with a faulty immune system, due to a genetic mutation. That makes them well suited for transplants, since their immune system is less likely to reject the implanted human cells. But it also means that studies of neurodegenerative diseases like Alzheimer’s that are known to have immune components may be more difficult. And no matter how realistic the transplanted human brain organoids get, as long as they’re in a rat brain, they will be exposed to rat blood, with its unique profile of nutrients and hormones, rather than human blood. Neuroscientists may thus be studying systems that fall somewhat short of the reality within the human skull.
But for Paşca, this new system offers the opportunity to get closer than ever to the ground truth about how altered neurobiological processes cause neurological and psychiatric disorders. Transplanting organoids into newborn rats finally offers a way to employ the full strength of modern neuroscience tools in research on the development of human neurons and circuits.
“Difficult problems, such as understanding psychiatric disorders that are uniquely human conditions, will require bold approaches,” said Paşca.