Longevity Linked to Proteins That Calm Overexcited Neurons

Higher levels of neural excitation are linked to shorter life spans in people and other animals, according to recent studies.
Wenyi Geng for Quanta Magazine
Introduction
A thousand seemingly insignificant things change as an organism ages. Beyond the obvious signs like graying hair and memory problems are myriad shifts both subtler and more consequential: Metabolic processes run less smoothly; neurons respond less swiftly; the replication of DNA grows faultier.
But while bodies may seem to just gradually wear out, many researchers believe instead that aging is controlled at the cellular and biochemical level. They find evidence for this in the throng of biological mechanisms that are linked to aging but also conserved across species as distantly related as roundworms and humans. Whole subfields of research have grown up around biologists’ attempts to understand the relationships among the core genes involved in aging, which seem to connect highly disparate biological functions, like metabolism and perception. If scientists can pinpoint which of the changes in these processes induce aging, rather than result from it, it may be possible to intervene and extend the human life span.
So far, research has suggested that severely limiting calorie intake can have a beneficial effect, as can manipulating certain genes in laboratory animals. But recently in Nature, Bruce Yankner, a professor of genetics and neurology at Harvard Medical School, and his colleagues reported on a previously overlooked controller of life span: the activity level of neurons in the brain. In a series of experiments on roundworms, mice and human brain tissue, they found that a protein called REST, which controls the expression of many genes related to neural firing, also controls life span. They also showed that boosting the levels of the equivalent of REST in worms lengthens their lives by making their neurons fire more quietly and with more control. How exactly overexcitation of neurons might shorten life span remains to be seen, but the effect is real and its discovery suggests new avenues for understanding the aging process.
Genetic Mechanisms of Aging
In the early days of the molecular study of aging, many people were skeptical that it was even worth looking into. Cynthia Kenyon, a pioneering researcher in this area at the University of California, San Francisco, has described attitudes in the late 1980s: “The ageing field at the time was considered a backwater by many molecular biologists, and the students were not interested, or were even repelled by the idea. Many of my faculty colleagues felt the same way. One told me that I would fall off the edge of the Earth if I studied ageing.”
That was because many scientists thought that aging (more specifically, growing old) must be a fairly boring, passive process at the molecular level — nothing more than the natural result of things wearing out. Evolutionary biologists argued that aging could not be regulated by any complex or evolved mechanism because it occurs after the age of reproduction, when natural selection no longer has a chance to act. However, Kenyon and a handful of colleagues thought that if the processes involved in aging were connected to processes that acted earlier in an organism’s lifetime, the real story might be more interesting than people realized. Through careful, often poorly funded work on Caenorhabditis elegans, the laboratory roundworm, they laid the groundwork for what is now a bustling field.
A key early finding was that the inactivation of a gene called daf-2 was fundamental to extending the life span of the worms. “daf-2 mutants were the most amazing things I had ever seen. They were active and healthy and they lived more than twice as long as normal,” Kenyon wrote in a reflection on these experiments. “It seemed magical but also a little creepy: they should have been dead, but there they were, moving around.”
This gene and a second one called daf-16 are both involved in producing these effects in worms. And as scientists came to understand the genes’ activities, it became increasingly clear that aging is not separate from the processes that control an organism’s development before the age of sexual maturity; it makes use of the same biochemical machinery. These genes are important in early life, helping the worms to resist stressful conditions during their youth. As the worms age, modulation of daf-2 and daf-16 then influences their health and longevity.
These startling results helped draw attention to the field, and over the next two decades many other discoveries illuminated a mysterious network of signal transduction pathways — where one protein binds another protein, which activates another, which switches off another and so on — that, if disturbed, can fundamentally alter life span. By 1997, researchers had discovered that in worms daf-2 is part of a family of receptors that send signals triggered by insulin, the hormone that controls blood sugar, and the structurally similar hormone IGF-1, insulin-like growth factor 1; daf-16 was farther down that same chain. Tracing the equivalent pathway in mammals, scientists found that it led to a protein called FoxO, which binds to the DNA in the nucleus, turning a shadowy army of genes on and off.
That it all comes down to the regulation of genes is perhaps not surprising, but it suggests that the processes that control aging and life span are vastly complex, acting on many systems at once in ways that may be hard to pick apart. But sometimes, it’s possible to shine a little light on what’s happening, as in the Yankner group’s new paper.
Get Plenty of REST
Figuring out which genes are turned on and off in aging brains has long been one of Yankner’s interests. About 15 years ago, in a paper published in Nature, he and his colleagues looked at gene expression data from donated human brains to see how it changes over a lifetime. Some years later, they realized that many of the changes they’d seen were caused by a protein called REST. REST, which turns genes off, was mainly known for its role in the development of the fetal brain: It represses neuronal genes until the young brain is ready for them to be expressed.
But that’s not the only time it’s active. “We discovered in 2014 that [the REST gene] is actually reactivated in the aging brain,” Yankner said.
To understand how the REST protein does its job, imagine that the network of neurons in the brain is engaged in something like the party game Telephone. Each neuron is covered with proteins and molecular channels that enable it to fire and pass messages. When one neuron fires, it releases a flood of neurotransmitters that excite or inhibit the firing of the next neuron down the line. REST inhibits the production of some of the proteins and channels involved in this process, reining in the excitation.
In their new study, Yankner and his colleagues report that the brains of long-lived humans have unusually low levels of proteins involved in excitation, at least in comparison with the brains of people who died much younger. This finding suggests that the exceptionally old people probably had less neural firing. To investigate this association in more detail, Yankner’s team turned to C. elegans. They compared neural activity in the splendidly long-lived daf-2 mutants with that of normal worms and saw that firing levels in the daf-2 animals were indeed very different.
“They were almost silent. They had very low neural activity compared to normal worms,” Yankner said, noting that neural activity usually increases with age in worms. “This was very interesting, and sort of parallels the gene expression pattern we saw in the extremely old humans.”
When the researchers gave normal roundworms drugs that suppressed excitation, it extended their life spans. Genetic manipulation that suppressed inhibition — the process that keeps neurons from firing — did the reverse. Several other experiments using different methods confirmed their results. The firing itself was somehow controlling life span — and in this case, less firing meant more longevity.
Because REST was plentiful in the brains of long-lived people, the researchers wondered if lab animals without REST would have more neural firing and shorter lives. Sure enough, they found that the brains of elderly mice in which the Rest gene had been knocked out were a mess of overexcited neurons, with a tendency toward bursts of activity resembling seizures. Worms with boosted levels of their version of REST (proteins named SPR-3 and SPR-4) had more controlled neural activity and lived longer. But daf-2 mutant worms deprived of REST were stripped of their longevity.
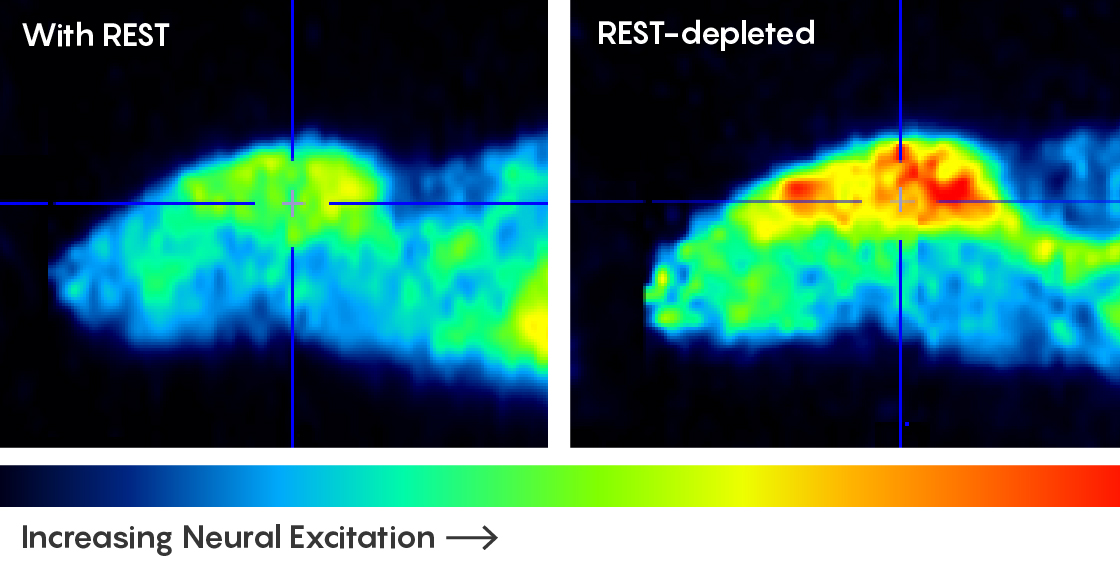
PET scans of mice (in profile) reveal that in animals with deficiencies of the REST protein, levels of neural excitation are higher.
“It suggests that there is a conserved mechanism from worms to [humans],” Yankner said. “You have this master transcription factor that keeps the brain at what we call a homeostatic or equilibrium level — it doesn’t let it get too excitable — and that prolongs life span. When that gets out of whack, it’s deleterious physiologically.”
What’s more, Yankner and his colleagues found that in worms the life extension effect depended on a very familiar bit of DNA: daf-16. This meant that REST’s trail had led the researchers back to that highly important aging pathway, as well as the insulin/IGF-1 system. “That really puts the REST transcription factor somehow squarely into this insulin signaling cascade,” said Thomas Flatt, an evolutionary biologist at the University of Fribourg who studies aging and the immune system. REST appears to be yet another way of feeding the basic molecular activities of the body into the metabolic pathway.
A Biological Balancing Act
Neural activity has been implicated in life span before, notes Joy Alcedo, a molecular geneticist at Wayne State University who studies the connections between sensory neurons, aging and developmental processes. Previous studies have found that manipulating the activity of even single neurons in C. elegans can extend or shorten life span. It’s not yet clear why, but one possibility is that the way the worms respond biochemically to their environment may somehow trip a switch in their hormonal signaling that affects how long they live.
The new study, however, suggests something broader: that overactivity in general is unhealthy. Neuronal overactivity may not feel like anything in particular from the viewpoint of the worm, mouse or human, unless it gets bad enough to provoke seizures. But perhaps over time it may damage neurons.
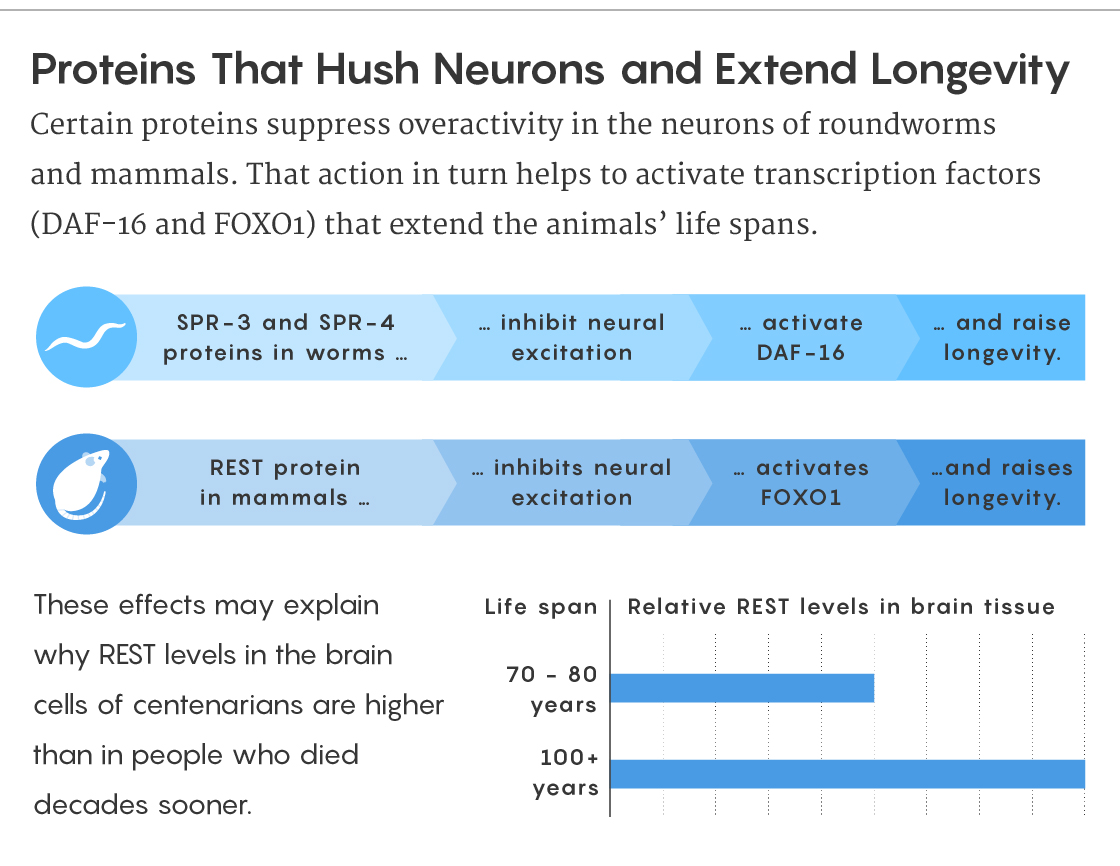
Lucy Reading-Ikkanda/Quanta Magazine; data source: doi:10.1038/s41586-019-1647-8
The new work also ties into the idea that aging may fundamentally involve a loss of biological stability, Flatt said. “A lot of things in aging and life span somehow have to do with homeostasis. Things are being maintained in a proper balance, if you will.” There’s a growing consensus in aging research that what we perceive as the body slowing down may in fact be a failure to preserve various equilibria. Flatt has found that aging flies show higher levels of immune-related molecules, and that this rise contributes to their deaths. Keeping the levels in check, closer to what they might have been when the flies were younger, extends their lives.
The results may help explain the observation that some drugs used for epilepsy extend life span in lab animals, said Nektarios Tavernarakis, a molecular biologist at the University of Crete who wrote a commentary that accompanied Yankner’s recent paper. If overexcitation shortens life span, then medicines that systematically reduce excitation could have the opposite effect. “This new study provides a mechanism,” he said.
In 2014, Yankner’s laboratory also reported that patients with neurodegenerative diseases like Alzheimer’s have lower levels of REST. The early stages of Alzheimer’s, Yankner notes, involve an increase in neural firing in the hippocampus, a part of the brain that deals with memory. He and his colleagues wonder whether the lack of REST contributes to the development of these diseases; they are now searching for potential drugs that boost REST levels to test in lab organisms and eventually patients.
In the meantime, however, it’s not clear that people can do anything to put the new findings about REST to work in extending their longevity. According to Yankner, REST levels in the brain haven’t been tied to any particular moods or states of intellectual activity. It would be a “misconception,” he explained by email, “to correlate amount of thinking with life span.” And while he notes that there is evidence that “meditation and yoga can have a variety of beneficial effects for mental and physical health,” no studies show that they have any bearing on REST levels.
Why exactly do overexcited neurons lead to death? That’s still a mystery. The answer probably lies somewhere downstream of the DAF-16 protein and FoxO, in the genes they turn on and off. They may be increasing the organism’s ability to deal with stress, reworking its energy production to be more efficient, shifting its metabolism into another gear, or performing any number of other changes that together add up a sturdier and longer-lived organism. “It is intriguing that something as transient as the activity state of a neural circuit could have such a major physiological influence on something as protean as life span,” Yankner said.
This article was reprinted on Wired.com.



