RNA’s Secret Life Outside the Cell
For decades, researchers have been finding DNA and its sister, RNA, circulating in the body, outside the safe interior of cells where these molecules do their essential work of storing and translating the code of life. The reasons for these molecular voyages have remained mysterious, but in recent years evidence has accrued that this extracellular RNA may have a different job, at least in some organisms.
RNA, best known to basic biology students for its role in translating genes into proteins, has turned out to be a surprisingly versatile and cosmopolitan molecule. Plants, roundworms, flatworms and insects use RNA to carry signals through their tissues, and perhaps further. Inspired by laboratory studies hinting that RNA may play a role in interactions between organisms, and even different species, Eric Miska, a molecular geneticist at the University of Cambridge, coined the term “social RNA” to describe the molecule’s apparent role in communication both inside and outside organisms.
Plants and the pests seeking to infect them can deploy RNA against one another. In a paper published in Science in October, researchers describe how a fungus — one responsible for both destroying crops with gray mold and producing the noble rot that flavors dessert wines — protects itself by using its own small RNA molecules to hijack the plants’ RNA defense machinery, silencing genes that would normally fight fungal infections. Discoveries like this point to a role for RNA in the arms race between plants and parasites, one of the potential instances of social RNA, Miska said. “I think it’s quite exciting, but it is early days,” Miska said. “A lot of things need to be discovered yet.”
While RNA’s role in signaling in plants and invertebrates is not fully understood, that role is clearly established. This is not the case for RNA in mammals, including humans. In these species, scientists know these molecules are traveling outside cells, but it is not yet clear whether or not they are a form of communication.
RNA has been found in a panoply of human body fluids: blood, urine, tears, cerebrospinal fluid, breast milk, amniotic fluid, seminal fluid and others. Moreover, scientists have discovered that small bits of circulating RNA can reflect particular conditions, such as the presence of a cancerous tumor or pregnancy-related disorders. “It’s like opening up a Pandora’s box,” said Xandra Breakefield, a neurogeneticist at Massachusetts General Hospital, of the discovery of circulating RNA. “We didn’t realize all these things were out there.”
While some remain skeptical that extracellular RNA and DNA are anything more than debris, Breakefield and others see a much more exciting prospect: that these might be a newly discovered form of communication among cells that plays a role in human health. For example, some studies suggest that small RNAs act as instructions that help coordinate an immune response or prep cancer cells to invade healthy tissue.
A Silencing Signal
Beginning in the late 1950s, RNA (ribonucleic acid) was cast as a servant to its higher-profile sister DNA (deoxyribonucleic acid), a role that turned out to involve transcribing the genetic code and assembling it into the proteins that build cells and enable them to function. In recent decades, however, RNA’s job description has expanded: It can kick-start chemical reactions, regulate the activity of genes within a cell and now, some suggest, serve as a signal that allows one cell to influence the behavior of others.
About 15 years ago, researchers figured out they could make the roundworm Caenorhabditis elegans twitch by injecting it with complementary strands of RNA that matched the sequence of a gene responsible for a protein in muscle fiber. The arrival of this double-stranded RNA sets off a process that effectively turns off the target gene and, in this case, damages the worm’s muscles.
Scientists have since discovered this type of RNA silencing in many organisms. They believe it helps defend against infection by shutting down the activity of invading viruses, which can temporarily exist as double-stranded RNA. When this double-stranded RNA pops up inside a worm cell, the worm’s molecular machinery uses it as a guide to shut off the viral genes that produced it. This process is called RNA interference, and it also generates an RNA silencing signal that spreads through the worm via a molecular channel. Similar signals have been shown to spread through the bodies of insects, flatworms and plants.
Evidence for social RNA in plants and invertebrates inevitably raises the question: What about us? Like plants and invertebrates, mammals are capable of silencing genes through RNA interference, but this system does not appear to play a major role in our immune system. So far, there is no evidence that mammalian cells can broadcast an RNA silencing signal as worm cells do. But some suspect a separate type of RNA, called microRNA, plays a similar social role in mammals.
Viral Invasion
Plants and invertebrates respond to a potential viral invasion by shutting down the viral genes using a process called RNA interference (RNAi). Mammals, including humans, have the molecular machinery to produce an RNAi response, but they do not appear to use it to defend themselves, relying instead on other defense mechanisms. However, two studies published Oct. 11 in the journal Science suggest mammals can fight viruses with RNAi. In one case, researchers took away a virus’s defense against RNAi, which it was known to use when infecting fruit flies. Normally, the virus kills young mice. But the mice could clear the infection with crippled virus, presumably thanks to RNAi. In the other study, researchers altered mouse embryonic stem cells so they could not produce an enzyme necessary for RNAi. As a result, the cells no longer produced RNA molecules implicated in an RNAi response. However, scientists say this is likely a minor antiviral mechanism in mammals. In plants and invertebrates, the gene silencing signal produced by RNAi can spread from cell to cell. There is no evidence this occurs in mammals.
The microRNA pathway is related to the RNA interference pathway, but microRNAs differ from the molecules involved in RNA interference in a couple of significant ways: MicroRNAs are encoded in the genome and regulate other genes in the same organism. Unlike RNA interference, which silences the genes of an infecting virus, microRNAs turn down expression of genes within the cell in which they are produced.
While the role microRNAs play inside cells is well understood, it is not clear why they are floating around outside them. Some mammalian cells spit out intercellular packages, called vesicles, that are taken up by other cells. In 2007, researchers discovered that mammalian cells can insert RNA, including microRNAs, into these packages. The findings suggest a novel way for one cell to influence the activity of another.
“We know that some cells put a lot of specific RNAs into these vesicles,” Breakefield said. “They are definitely just gobbled up [by other cells], so there is the potential to transfer information in this way.”
It has since turned out that a menagerie of RNAs, other molecules and even pieces of DNA can be found tucked into vesicles, and that vesicles are not microRNA’s only ride. The molecule can circulate through the body bound to proteins, which protect it from the hostile environment outside the cell, and by other means as well.
Evidence and Uncertainty
To understand what circulating microRNAs are up to, scientists must confirm that these molecules are indeed transferred from one cell to another. Because cells produce many microRNAs, it can be difficult to determine where a given microRNA originated. To solve this problem, D. Michiel Pegtel, a cell biologist at VU University Medical Center in Amsterdam, and colleagues turned to a virus, Epstein-Barr. The virus forces infected cells to produce viral microRNAs that help the virus replicate. Since no normal cell would produce viral microRNAs, these are relatively easy to track.
Pegtel and colleagues started with two types of immune cells; B cells, a type of white blood cell, infected with the virus, and dendritic cells, which sense viral invaders and alert other immune cells. The two were separated by a membrane with pores small enough to allow only vesicles to pass through.
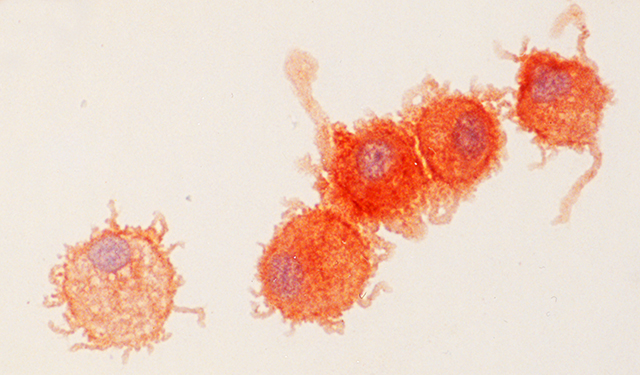
The dendritic cells were genetically engineered to glow until microRNAs that the virus had forced the B cells to produce traveled across the barrier and quieted the glowing genes. The results, published in the Proceedings of the National Academy of Sciences in 2010, show that the transfer of the vesicles across the membrane does indeed dim the glowing cells.
However, not everyone is convinced. The results from this and other RNA transfer experiments likely have other explanations, said Thomas Tuschl, a nucleic acid chemist and biochemist at Rockefeller University. The fusion of the vesicle with the cell resembles a viral infection. So Tuschl suspects that something about the fusion process, or perhaps something inside the vesicle, which can carry many different types of molecules, could trigger an immune response within the cell. This in turn could spark changes in the cells that resemble the supposed effect of the arriving RNA, Tuschl said.
Pegtel said that is unlikely. An extra test showed that the viral RNAs would target one of the virus’s own genes if they were placed in the dendritic cell. What’s more, the degree of dimming in the glowing dendritic cells corresponded to the amount of viral RNA-bearing vesicles that bombarded them, he said. Vesicles lacking viral microRNA did not show the dimming effect.
Nonetheless, Tuschl is skeptical of microRNA’s role in intercellular signaling in mammals for other reasons as well. These small RNAs are present at low concentrations, and mammals, unlike plants and invertebrates, have no significant mechanism to amplify an RNA signal. “In general, there is too little of everything to make this an effective signaling mechanism,” Tuschl said.
Others are skeptical too. Mark Kay, a geneticist at Stanford School of Medicine, doesn’t dismiss the possibility that extracellular microRNA serves this purpose, but he is not ready to embrace it. “I try to keep an open mind, but I don’t think it is convincing at this point that the signaling is occurring in mammalian systems,” Kay said.
Even Pegtel is cautious, saying that scientists have a way to go before they can state definitively that circulating RNA causes specific changes upon arriving in cells. Most of the mammalian studies to date have been done in cells growing in test tubes rather than in living mammals. As Pegtel pointed out, these experiments rely on unnatural conditions, such as highly concentrated doses of vesicles and microRNA. He said, “That effect is very artificial.”
The next step, he said, will be to try to show that vesicle-borne RNA has a meaningful effect within the immense complexity of living mammals. “Time will tell.”
A new round of experiments could help to answer the questions and to clarify the role of circulating RNA in human health and disease. The National Institutes of Health announced in August $17 million in funds for 24 research projects focused on understanding extracellular RNA, including microRNA, and using these molecules to diagnose and treat disease.
Breakefield, who received one of the grants, is examining how RNA released by glioblastoma, a highly aggressive form of brain cancer, manipulates surrounding cells to support its own growth. Tuschl, also a grantee, is exploring RNA’s potential use as a marker for autoimmune disease. Through a separate grant, he also hopes to study a potential alternative explanation for the changes in cells that follow the arrival of the RNA-bearing vesicles.
From the NIH’s perspective, evidence already suggests this RNA can act as a signal. But even if traveling RNAs are only debris, they might still have uses as markers for disease and as a means to conscript the vesicles that carry them to deliver drugs to hard-to-reach places, said Danilo Tagle, associate director for special initiatives at the National Center for Advancing Translational Sciences, which is involved in the NIH’s extracellular RNA program.
The implications for cell biology and medicine are overarching, Tagle said. “In a sense we are opening up a new area of research,” he said.
This article was reprinted on Wired.com.