How Two Became One: Origins of a Mysterious Symbiosis Found

A queen of the carpenter ant species Camponotus sansabeanus with her eggs. Essential bacteria passed down in the eggs guide the ants’ early embryonic development and sustain them later in life.
Introduction
Symbiotic relationships between bacteria and multicellular organisms are everywhere in nature, but some are more intricately intertwined than others. Both cows and carpenter ants, for example, rely on bacterial partners in their digestive systems to help them get the most out of their food. Yet while the cows’ bacteria merely inhabit the animals’ stomach, the bacteria in the ants live inside their gut cells as endosymbionts.
Understanding endosymbiosis is a prize puzzle for biologists because it is so central to life as we know it: Mitochondria, the organelles that power all complex cells, are the remnants of a very ancient endosymbiotic event. Yet explaining how such intense interdependencies between species evolve is always a challenge. Even the mechanisms that reliably guarantee the endosymbionts will get into the right cells of their hosts and be passed from one generation to the next can be mysterious.
Last week in Nature, however, a trio of scientists working at McGill University announced their discovery of how the essential endosymbiosis in carpenter ants evolved and helped them become some of the most successful creatures on the planet. What the researchers pieced together is that this seemingly harmonious partnership evolved through a duel at the cellular and genetic levels, one that left the ant eggs largely unviable on their own. The bacteria have seized genetic control over crucial stages of the ant’s early development and literally reshaped the embryo into a vessel for their own survival.
“The microbe in this case has essentially co-opted aspects of the host’s own cellular system in order to benefit itself,” commented Corrie Moreau, an evolutionary biologist at Cornell University.
Daniel Kronauer of the Rockefeller University, who studies the evolution of insect societies and was not part of the study, hailed the work as “quite a technical tour de force” in an email to Quanta. “The study provides beautiful insights into how a complex evolutionary interaction between vastly different partners can play out on a molecular and developmental level over evolutionary time.”
Partnering for Success
Of the roughly 12,000 known species of ants in the world, more than 1,000 are carpenter ants, in the hyperdiverse genus Camponotus. That genus and seven other related living ones make up the Camponotini ant “tribe,” all of which have bacteria inside their gut cells. The bacteria are classified as obligate endosymbionts, meaning they need the ants as hosts as much as the ants need them.
These endosymbionts were one of the first ever described in an animal. In 1882, the German zoologist Friedrich Blochmann discovered the microorganisms in carpenter ant embryos. He mistakenly called them fungi, but scientists later identified the bright cloud of DNA in the embryo’s posterior pole as bacteria and named them Blochmannia after their discoverer. In adult carpenter ants, the bacteria produce essential amino acids and play important roles in immunity. In exchange, the carpenter ants provide a protective cellular environment for the Blochmannia and transmit them to their offspring, ensuring the bacteria’s survival.
How the endosymbiont got into the ants is unclear, but evidence from genetics and ecology hints that the endosymbiont was horizontally transferred into an ancestor of Camponotus about 51 million years ago from sap-feeding insects that the ants protected in exchange for sugary secretions. The ants and the bacteria then forged a partnership that now develops, reproduces and evolves as a single unit. To learn how that was possible, a team of researchers in the laboratory of Ehab Abouheif at McGill University in Montreal started at the very beginning: by looking at freshly laid ant eggs.
Developmental Gymnastics
Insect eggs and embryos behave rather differently from those of chickens and other vertebrates during the early stages of development: The activated nucleus replicates over and over, but the single egg cell doesn’t split for quite some time. Instead of becoming a ball of distinct cells, the insect egg forms a syncytium, a single huge multinucleate cell. The structure of the embryo emerges as the nuclei and other cellular materials gradually arrange themselves within this mass. Only later are the cytoplasm and its contents partitioned into different cells.
Messenger RNA molecules passed down from the mother (maternal mRNAs) are positioned at the ends of the embryo and are used to establish an axis for the body plan. Then the embryo takes over and a strict sequence of embryonic genes kicks in, setting up more features. At the end of that chain, Hox genes turn on to specify the insect’s head, thorax and abdomen.
When the McGill researchers scrutinized the development of carpenter ant embryos, however, they saw to their surprise that Hox proteins were appearing during the first nuclear divisions — far ahead of schedule. The proteins were being made from maternal mRNAs in the cytoplasm, “which was something that we would not have expected from any of the data we know from any other insects,” said Arjuna Rajakumar, a graduate student in Abouheif’s lab and a co-author of the study.
By comparing the embryos of 31 ant species, the scientists deduced that this quirk of expressing maternal Hox mRNAs very early in development must have evolved in ancestors of the Camponotini ant tribe — and long before endosymbionts started living inside ants.
Why those ancestral ants started developing this way is unknown, but it had an important consequence: It eventually enabled the ancestors of Camponotini ants to pick up an endosymbiont partner. “The key finding of this paper is that in order for these insects to engage in this endosymbiosis, they already had to have these pre-existing developmental systems in place,” Moreau said.
And once the endosymbiont was inside the ants, major features of the insects’ embryonic development changed dramatically, apparently through a series of moves and countermoves by both species to control the arrangement. This became clear when the researchers looked more closely at what happens to the germline in carpenter ants, the tissues that produce eggs and sperm in the sexually mature adults.

Ehab Abouheif (left), a professor of evolutionary and developmental biology at McGill University, and Arjuna Rajakumar, a postdoctoral researcher in his laboratory.
Normally, ant eggs have a single zone at their posterior pole that expresses germline genes. But the scientists saw that in eggs of the carpenter ant C. floridanus, there are four of these zones. The original zone at the posterior pole, which Blochmann had seen, teems with bacteria. It is as if the ancestral germline was “hijacked” by the bacteria, explains Matteen Rafiqi, who was a postdoctoral scientist in Abouheif’s lab and now leads his own lab at Bezmialem Vakif University in Istanbul.
This hijacking makes evolutionary sense, given that the bacteria enter the germline to ensure their vertical transmission. But if too many bacteria ended up in the germline cells, it could undermine the ants’ own genetic integrity. So the ants evolved to leave the ancestral germline as a “decoy” to attract the bacteria, Rajakumar said. Instead of getting into the germline, the Blochmannia are enclosed in specialized structures called bacteriocytes — honeycomb-shaped ant cells teeming with spaghettilike bacteria — and transported to the larval gut, where they can eventually aid the ants’ digestive needs.
The ant also created two additional germline zones: one to produce its germline tissues and one to help guide the bacteriocyte package to the gut. Only a few bacteria make it into the true germline, but it’s enough to guarantee transmission to the next generation of ants.
“The way I see it is that the bacteria are paying their dues to the host,” Abouheif said.
But the bacteria may have the last laugh after all, as the researchers discovered when they exposed the ant embryos to an antibiotic to eliminate Blochmannia.
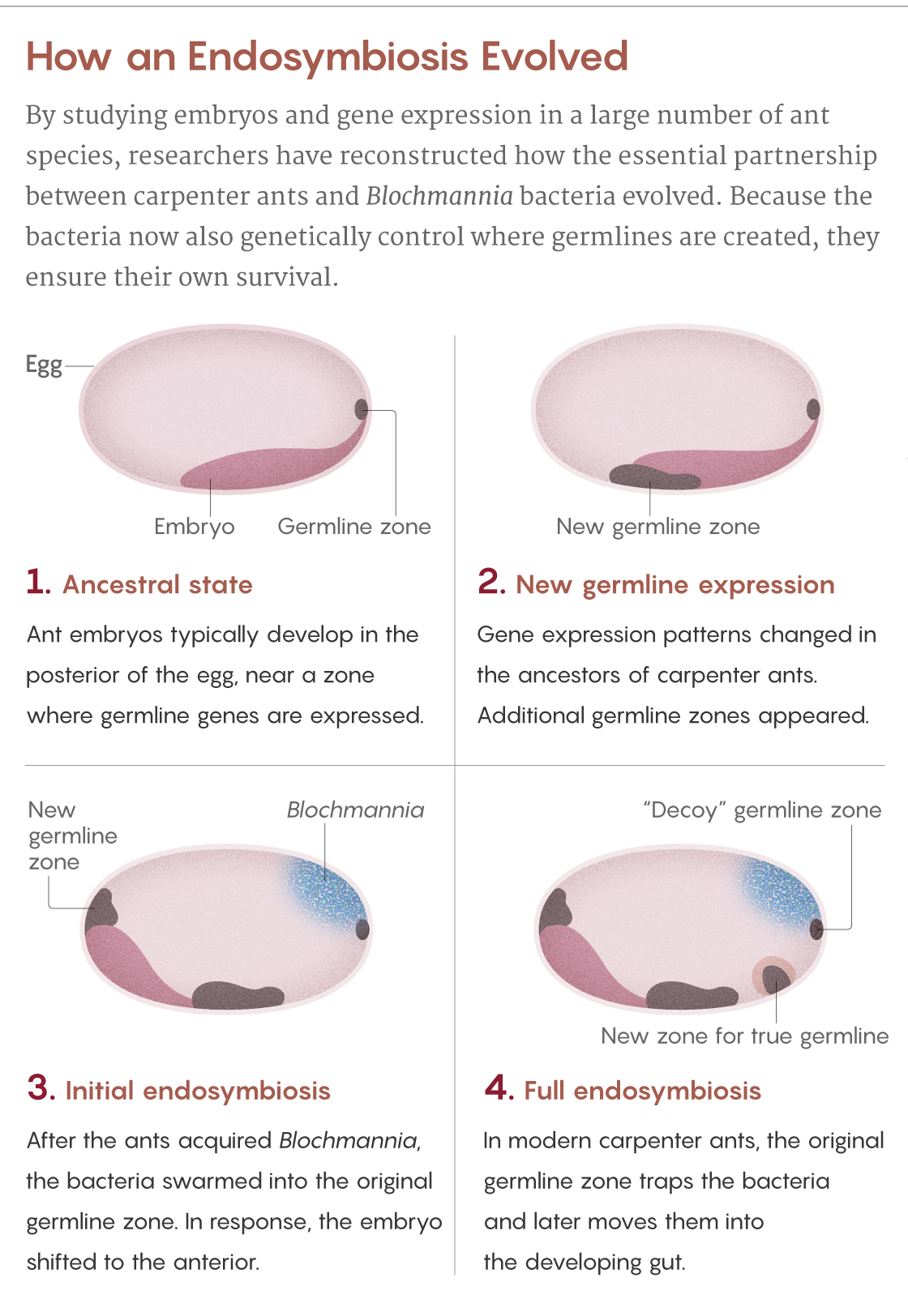
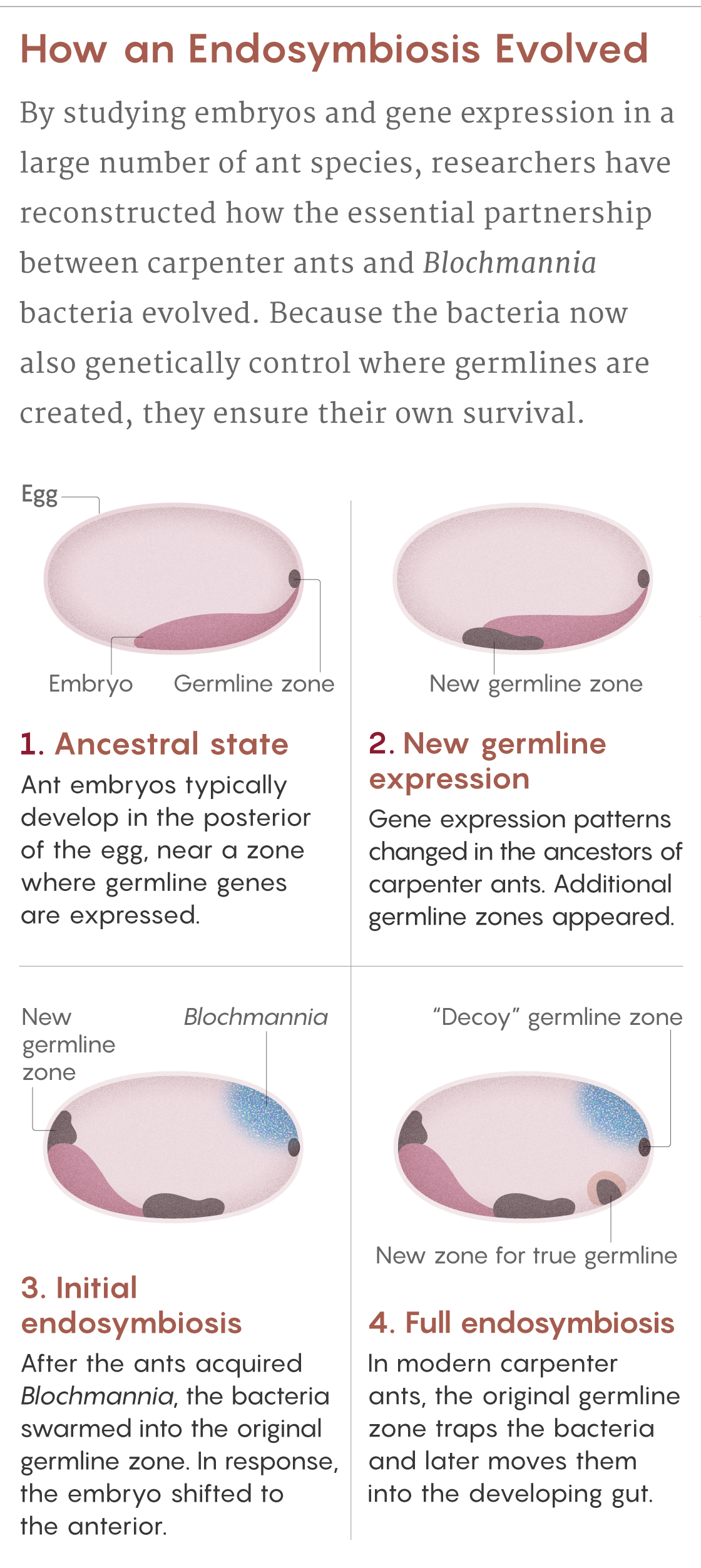
Samuel Velasco/Quanta Magazine; source: Rafiqi et al., doi: 10.1038/s41586-020-2653-6
Moves and Countermoves
Without endosymbionts, more than half the embryos failed to develop at all. Those that did hatch had defective germ cells. As embryos, they still had four zones of germline gene expression, but the subsets of genes expressed in each zone had changed. Those developing embryos were reverting into ants that looked more like their ancient endosymbiont-free ancestors.
The researchers concluded that the endosymbionts had taken over the job of instructing the ant embryo how to assemble some parts of itself. “What we found is that the bacteria is able to selectively regulate the expression of specific mRNAs and proteins,” Rajakumar said. “It is almost as if it was acting like a transcription factor.”
In particular, the bacteria seem to be steering aspects of ant development and physiology crucial to their endosymbiotic existence. For example, a number of insects use Hox genes to develop bacteriocytes for their symbiotic partners. But only in the Camponotini ant tribe do the endosymbionts turn on Hox genes in the developing embryo’s germline, because the endosymbiont needs to make a place for itself in what will become the egg and sperm cells. “The bacterium is actually controlling its own fate — it’s inducing its own vertical transmission,” Rajakumar said. “When you lose the bacteria, you actually lose the novel germline entirely.”
There may be an advantage for the ant, too, from rearranging its embryonic development to suit its partner: Packaging the bacteria into cells at that stage may be easier than later. “How else can you pack a gut cell with so many bacteria?” Abouheif asked. “You have to tinker with things during the syncytial stage” because then “you can coordinate where the bacteria are going to go.”
He added: “I have a hunch that there’s a universal constraint for obligate bacterial endosymbioses with animals. They have to do it at this stage.”
These discoveries raise the question of how host development could be altered in even more complex endosymbioses, when hosts are home to more than one partner. Various lineages of cicadas and leafhoppers, for example, have gained, lost or swapped multiple endosymbionts many times, according to Nancy Moran, who studies insect endosymbioses at the University of Texas at Austin. But “very few studies have looked at embryogenesis and how the symbionts are packaged, and how the regulation of development has shifted in order to incorporate the endosymbionts,” she said.
Abouheif thinks that what is happening in carpenter ants may illustrate a broader principle involving symbioses and evolution. The tweaking of intracellular localization mechanisms and “just messing around with” combinations of gene expression to generate new embryonic zones with different developmental fates “is a whole kind of mechanism of evolution that people don’t really think about,” he said.
Gradual Transitions, Evolutionary Leaps
Previous studies of endosymbiosis have shown that there can be complementary gene losses between hosts and endosymbionts, creating a metabolic interdependence. For instance, John McCutcheon at Arizona State University has looked at a complex example in mealybugs, whose cells contain two endosymbiotic microbes nested inside of each other. Over the last 100 million years, each endosymbiont evolved to rely on genes for enzymes in its partner’s genome. As a result, none of the partners can complete the metabolic pathway without the others.

Carpenter ants make a home for their larvae in some rotting wood.
But the study from Abouheif’s lab is the first to show that “the gene regulatory networks are getting developmentally intertwined,” Rafiqi said. “It’s as if the merger is complete.”
The researchers consider this merger an example of a “major evolutionary transition in individuality,” such as the transition from unicellular to multicellular organisms, the transition to eusociality in social insects, or the origin of mitochondria in eukaryotic cells. These transitions typically appear as discrete “leaps” in evolution, and the mechanisms that produce them remain little understood.
But this study, through its phylogenetic comparisons, was able to resolve the stepwise changes that in the ant lineage before and after the endosymbiosis occurred. “By looking at all the species in between, you start getting a much more gradual picture of how these things occur,” Rajakumar said.
“Comparative work is very important in endosymbiosis,” McCutcheon said. “You think it happens a certain way, and then you look at a sister group and it doesn’t work that way at all. The comparative approach here is really strong, and it helps the researchers time what was pre-existing before Blochmannia and what Blochmannia changed. That’s really exciting.”
The step-by-step reconstruction also showed that multiple conditions had to converge for the endosymbiosis to happen, Rafiqi said. Such events are usually considered rare. But when you evolve a complete transition in individuality like obligate endosymbiosis, “it ends up having a huge effect on the whole phyla,” Rajakumar said. “They can be evolutionary drivers, these bacteria.”
This article was reprinted in Italian at Lescienze.it.



