The Physics of Cold Water May Have Jump-Started Complex Life

Frigid temperatures hundreds of millions of years ago, during ancient periods known as Snowball Earth, may have created the conditions for complex multicellular life to evolve. New experiments show how.
Daniel García for Quanta Magazine
Introduction
Once upon a time, long ago, the world was encased in ice. That’s the tale told by sedimentary rock in the tropics, many geologists believe. Hundreds of millions of years ago, glaciers and sea ice covered the globe. The most extreme scenarios suggest a layer of ice several meters thick even at the equator.
This event has been called “Snowball Earth,” and you’d think it would be a terrible time to be alive — and maybe, for some organisms, it was. However, in a warmer period between glaciations, the first evidence of multicellular animals appears, according to some interpretations of the geological record. Life had taken a leap. How could the seeming desolation of a Snowball Earth line up with this burst of biological innovation?
A series of papers from the lab of Carl Simpson proposes an answer linked to a fundamental physical fact: As seawater gets colder, it gets more viscous, and therefore more difficult for very small organisms to navigate. Imagine swimming through honey rather than water. If microscopic organisms struggled to get enough food to survive under these conditions, as Simpson’s modeling work has implied, they would be placed under pressure to change — perhaps by developing ways to hang on to each other, form larger groups, and move through the water with greater force. Maybe some of these changes contributed to the beginning of multicellular animal life.
To test the idea, Simpson, a paleobiologist at the University of Colorado, Boulder, and his team conducted an experiment designed to see what a modern single-celled organism does when confronted with higher viscosity. Over the course of a month, he and his graduate student Andrea Halling watched how a type of green algae — members of a lab-friendly species that swims with a tail-like flagellum — formed larger, more coordinated groups as they encountered thicker gel. The algae collectively motored through the fluid to keep up their feeding pace. And, intriguingly, the groups of cells remained stuck together for 100 generations after the experiment ended.
The research offers a novel take on the emergence of multicellular life, said Phoebe Cohen, a paleontologist at Williams College who has spoken with Simpson about his idea over the years but was otherwise uninvolved with the work. The field is overflowing with papers about triggers for the evolution of animal multicellularity that draw on geochemical measurements, she said, but few consider the biology of individual organisms.

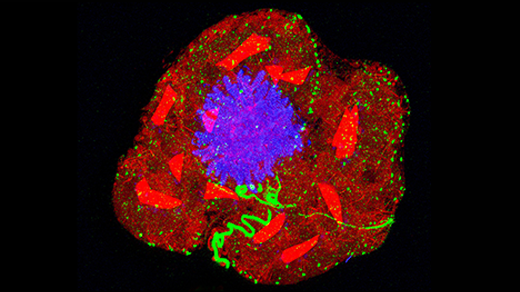
To re-create Snowball Earth conditions in the lab, biologists placed swimming algal cells into gel of varying viscosity. The cells that made it to the thickest, outer layer displayed signs of collective behavior — a potential step toward multicellularity.
Andrea Halling
“I’m very charmed by the idea, by the experimental setup as well,” Cohen said. “It’s really wonderful to see work saying: What’s actually going on here? How are these early organisms actually experiencing their environment?”
The experiment comes with a few caveats, and the paper has yet to be peer-reviewed; Simpson posted a preprint on biorxiv.org earlier this year. But it suggests that if Snowball Earth did act as a trigger for the evolution of complex life, it might be due to the physics of cold water.
A Frozen Paradox
“Snowball Earth” was on everyone’s lips when Simpson was an undergraduate in the late 1990s. In 1992, the geochemist Joseph Kirschvink had pointed out that there was good geological evidence for a global glaciation event in the ancient past; crucially, he provided a model for how all that ice might have been coerced to melt again. Then, in 1998, the Harvard geologist Paul Hoffman and colleagues published a landmark paper that applied these ideas to observations of sedimentary deposits in Namibia. They agreed: The rocks indicated the presence of glaciers in the warmest parts of the world around 700 million years ago.
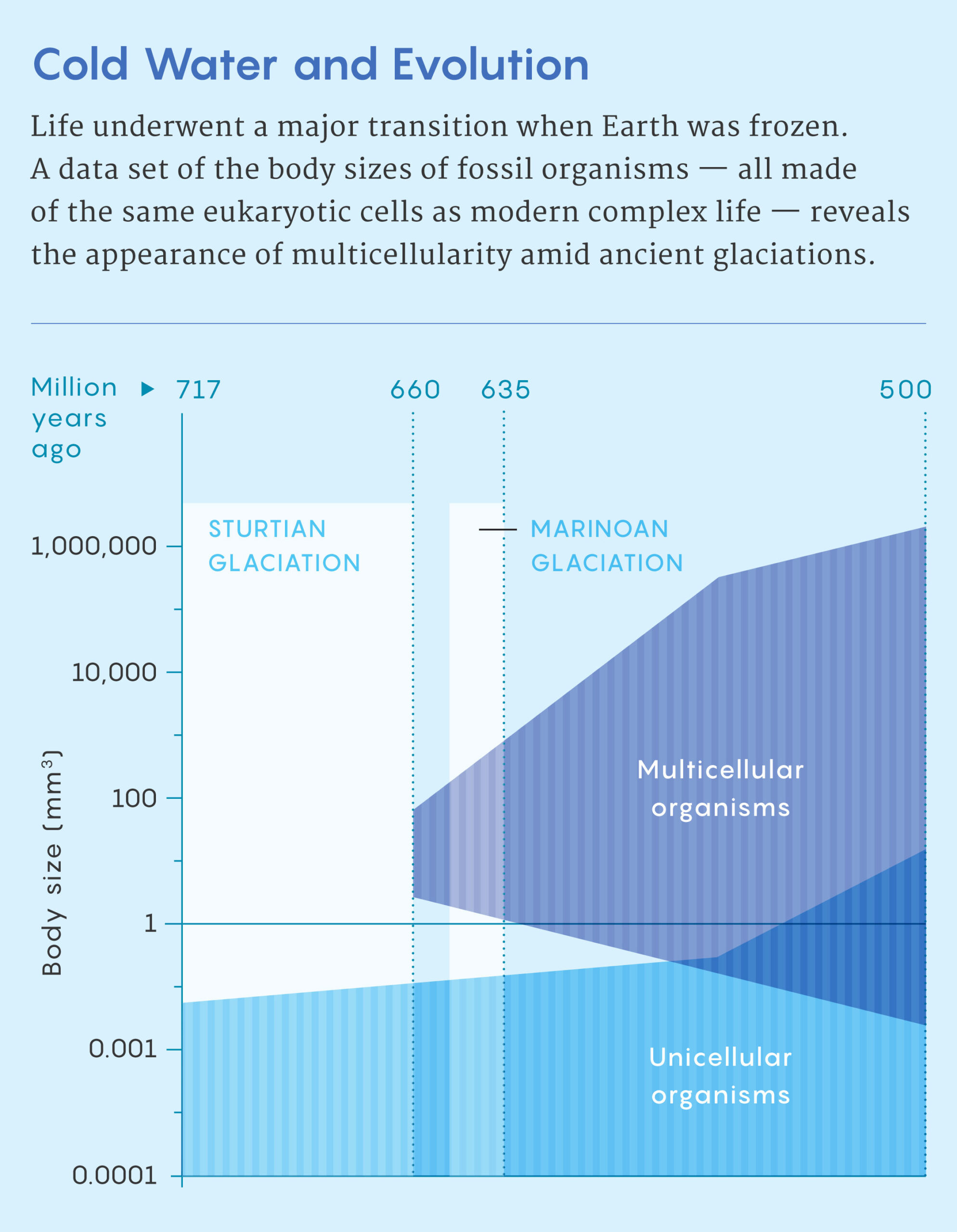
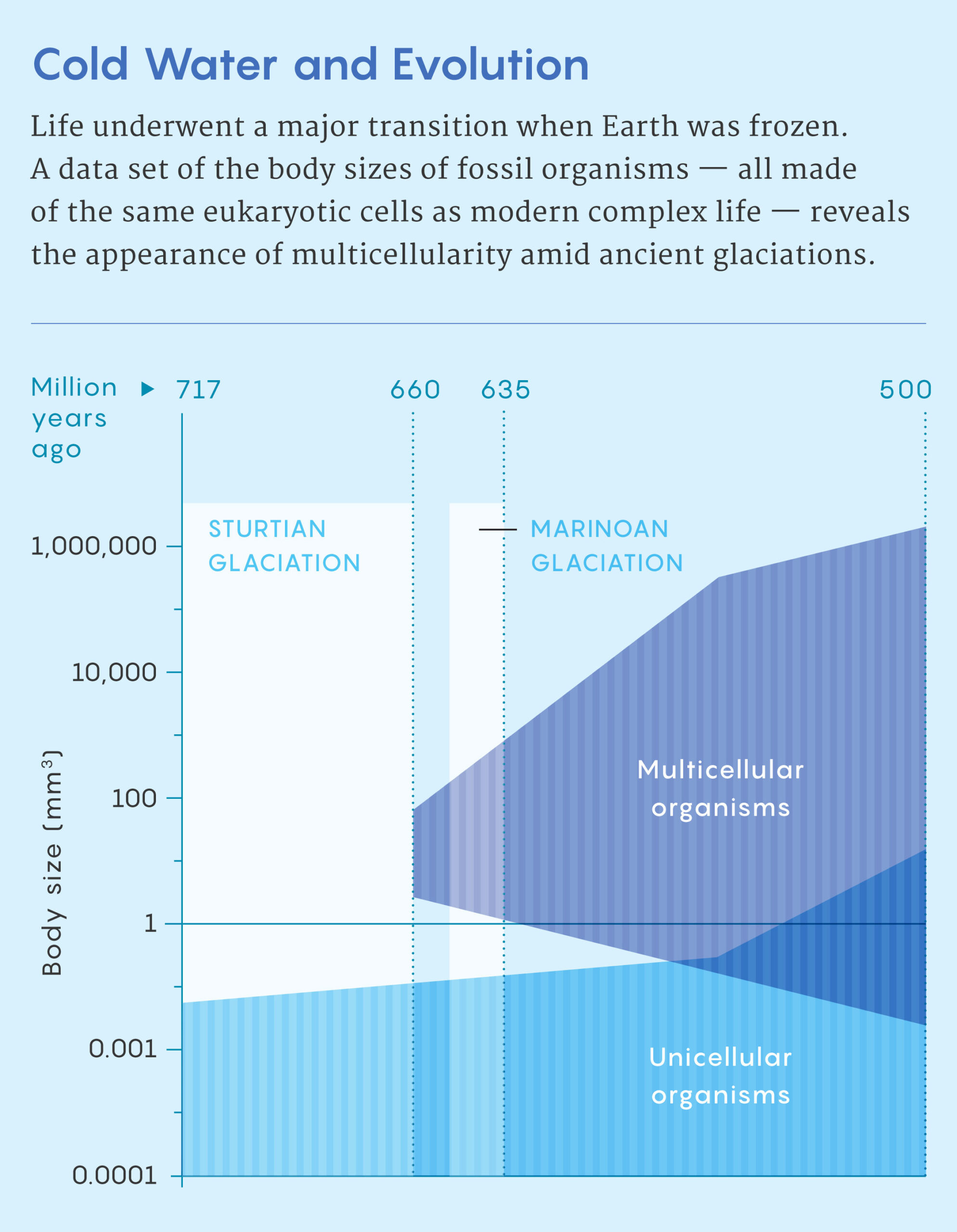
Even back then, the timing of Snowball Earth troubled Simpson. “That was a total paradox for me,” he said. “There’s no way Snowball Earth was real, given how much interesting evolution was happening at the time.” Before Snowball Earth, fossils are tiny, he said. Afterward, they are big and complicated.
It is difficult to precisely date when animals arose, but an estimate from molecular clocks — which use mutation rates to estimate the passage of time — suggests that the last common ancestor of multicellular animals emerged during the era known as the Sturtian Snowball Earth, sometime between 717 million and 660 million years ago. Large, unmistakably multicellular animals appear in the fossil record tens of millions of years after the Earth melted following another, shorter Snowball Earth period around 635 million years ago.
Mark Belan for Quanta Magazine
The paradox — a planet seemingly hostile to life giving evolution a major push — continued to perplex Simpson throughout his schooling and into his professional life. In 2018, as an assistant professor, he had an insight: As seawater gets colder, it grows thicker. It’s basic physics — the density and viscosity of water molecules rises as the temperature drops. Under the conditions of Snowball Earth, the ocean would have been twice or even four times as viscous as it was before the planet froze over.
Simpson wondered what it would have been like to be a microscopic organism in the ocean during Snowball Earth. Maybe the whole thing wasn’t so paradoxical after all.
To very small single-celled creatures, thick seawater would have posed some big problems. Bacteria feed by diffusion — the movement of nutrients through water from areas of high concentration to low concentration — and tend to wait for food to come to them. However, at low temperatures, diffusion slows down. Nutrients don’t travel as quickly or as far. For cells, living in a cold and more viscous fluid means getting less to eat. Even very small organisms that can propel themselves, such as cells with flagella, move more slowly in cold water. As a result, they encounter food less frequently.
A bigger organism, on the other hand, can navigate thicker waters without much trouble. A cluster of cells has the benefit of inertia: Their combined mass is large enough to allow them to build up steam and barrel through thicker fluid. “At some point, you are too big for this to matter,” Simpson said.
In 2021, he published his hypothesis that Snowball Earth viscosities would have put a significant strain on organisms’ ability to feed themselves and could have spurred some to evolve multicellularity. Then, with collaborators at the Santa Fe Institute, he designed mathematical models of small creatures — single cells that fed by diffusion and self-propelling cells that fed by moving around — living in thicker and thicker fluids. In the models, posted to biorxiv.org at the end of 2023 and recently published in the peer-reviewed Proceedings of the Royal Society B, the diffusion feeders responded to thicker fluids by shrinking in size. The self-propelling cells, equipped by the equations with the ability to cling together if needed, formed larger and larger multicellular groups. This suggested that if there were already multicellular organisms when Snowball Earth occurred — or at least organisms with the ability to take on multicellular forms — the thicker fluid could have given them a reason to get bigger.

The paleobiologist Carl Simpson has led a body of work — computer modeling and experiments with living organisms — to study whether the physics of cold water causes cells to act collectively like a multicellular creature.
Glenn Asakawa
The results were intriguing, but they were only computer models. Simpson thought: Well, what if they did this with real organisms?
The geologist Boswell Wing, a colleague at the University of Colorado, Boulder, had a colony of Chlamydomonas reinhardtii in his lab. These algae have twirling flagella that allow them to move under their own power. They are usually unicellular. But they can switch into a multicellular form under certain stressful conditions. Would higher viscosity, like that of the oceans during Snowball Earth, prove to be one of them?
Life in Thick Water
There’s no way for biologists to travel back in time to test the real conditions of Snowball Earth, but they can try to re-create aspects of them in the lab. In an enormous, custom-made petri dish, Halling and Simpson created a bull’s-eye target of agar gel — their own experimental gauntlet of viscosity. At the center, it was the standard viscosity used for growing these algae in the lab. Moving outward, each concentric ring had higher and higher viscosity, finally reaching a medium with four times the standard level. The scientists placed the algae in the middle, turned on a camera, and left them alone for 30 days — enough time for about 70 generations of algae to live, swim around for nutrients and die.
Halling and Simpson suspected that as the algae reproduced and crowded the center circle of normal viscosity, any algal cells that could handle the thicker medium would spread outward. Perhaps those that reached the outermost ring would look and behave differently from those that remained in the center.
Simpson was particularly curious as to whether algae that made it into the highest viscosity ring would find ways to increase their swimming speed. The algae are photosynthetic, so they get energy from the sun. But they need to pick up nutrients such as phosphorus from the environment, so movement is still important to their survival. Maintaining the same level of nutrients in high-viscosity surroundings would require them to find a way to keep up their speed.
After 30 days, the algae in the middle were still unicellular. As the scientists put algae from thicker and thicker rings under the microscope, however, they found larger clumps of cells. The very largest were wads of hundreds. But what interested Simpson the most were mobile clusters of four to 16 cells, arranged so that their flagella were all on the outside. These clusters moved around by coordinating the movement of their flagella, the ones at the back of the cluster holding still, the ones at the front wriggling.
Comparing the speed of these clusters to the single cells in the middle revealed something interesting. “They all swim at the same speed,” Simpson said. By working together as a collective, the algae could preserve their mobility. “I was really pleased,” he said. “With the coarse mathematical framework, there were a few predictions I could make. To actually see it empirically means there’s something to this idea.”
Intriguingly, when the scientists took these little clusters from the high-viscosity gel and put them back at low viscosity, the cells stuck together. They remained this way, in fact, for as long as the scientists continued to watch them, about 100 more generations. Clearly, whatever changes they underwent to survive at high viscosity were hard to reverse, Simpson said — perhaps a move toward evolution rather than a short-term shift.
Modern-day algae are not early animals. But the fact that these physical pressures forced a unicellular creature into an alternate way of life that was hard to reverse feels quite powerful, Simpson said. He suspects that if scientists explore the idea that when organisms are very small, viscosity dominates their existence, we could learn something about conditions that might have led to the explosion of large forms of life.
A Cell’s Perspective
As large creatures, we don’t think much about the thickness of the fluids around us. It’s not a part of our daily lived experience, and we are so big that viscosity doesn’t impinge on us very much. The ability to move easily — relatively speaking — is something we take for granted. From the time Simpson first realized that such limits on movement could be a monumental obstacle to microscopic life, he hasn’t been able to stop thinking about it. Viscosity may have mattered quite a lot in the origins of complex life, whenever that was.
“[This perspective] allows us to think about the deep-time history of this transition,” Simpson said, “and what was going on in Earth’s history when all the obligately complicated multicellular groups evolved, which is relatively close to each other, we think.”
Other researchers find Simpson’s ideas quite novel. Before Simpson, no one seems to have thought very much about organisms’ physical experience of being in the ocean during Snowball Earth, said Nick Butterfield of the University of Cambridge, who studies the evolution of early life. He cheerfully noted, however, that “Carl’s idea is fringe.” That’s because the vast majority of theories about Snowball Earth’s influence on the evolution of multicellular animals, plants and algae focus on how levels of oxygen, inferred from isotope levels in rocks, could have tipped the scales in one way or another, he said.
That novelty is a strength, said the geobiologist Jochen Brocks of the Australian National University. However, in his assessment, Simpson’s hypothesis makes a few logical leaps that don’t hold up. It’s not clear that the earliest animals would have been swimming freely in water, Brocks said. Some of the first fossils that can be confidently called “animals” were anchored on the ocean floor.
Perhaps more importantly, the timeline of animal origins is very uncertain. Some estimates suggest that the Snowball Earth period might line up with the last common ancestor of animals. But these are based on molecular inferences from DNA that are hard to confirm, Brocks said. In his opinion, it’s difficult to say how much importance to assign to this era. Butterfield also remarked on this uncertainty: “There’s no evidence of anything getting large until quite a bit after [Snowball Earth].”
That said, Brocks found Simpson’s experiment quite clever and beautiful. The fact that organisms might respond to high viscosity by developing collective behavior deserves to be better understood, he said — whether Snowball Earth led to the evolution of complex animal life or not.
“Putting this into our repertoire of thinking about why these things evolved — that is the value of the entire thing,” he said. “It doesn’t matter if it was Snowball Earth. It doesn’t matter if it happened before or after. Just the idea that it can happen, and happen quickly.”
Brocks is curious about what would happen if a similar experiment were performed with choanoflagellates, little creatures that are more closely related to animals than algae are. They rely entirely on hunting to get food — they can’t photosynthesize — so they would be especially vulnerable to slowdowns caused by high viscosity. If they started to take on multicellular forms under those conditions, that would suggest that Simpson’s results represent a more general truth about how life responds to its environment. “It would be absolutely ultra-exciting,” he said.
Simpson is, in fact, currently working with choanoflagellates. Right now, he is trying to understand how they live.
“They’re really beautiful and complicated creatures,” he said. They can take on many different forms: There are fast swimmers with long flagella, slow swimmers that meander, ones that stick to a surface to grow. “They can grow these little tendrils off the tip and walk around like on stilts; they have sex, and they fuse, and they form chain colonies and rosette colonies … and if you squeeze them, apparently they’ll lose their flagella and turn into an amoeba,” he said. When it comes to responding to the challenges of a radical new environment, he reflected, “they’ve got a lot to work with.”