Tight-Knit Microbes Live Together to Make a Vital Nutrient
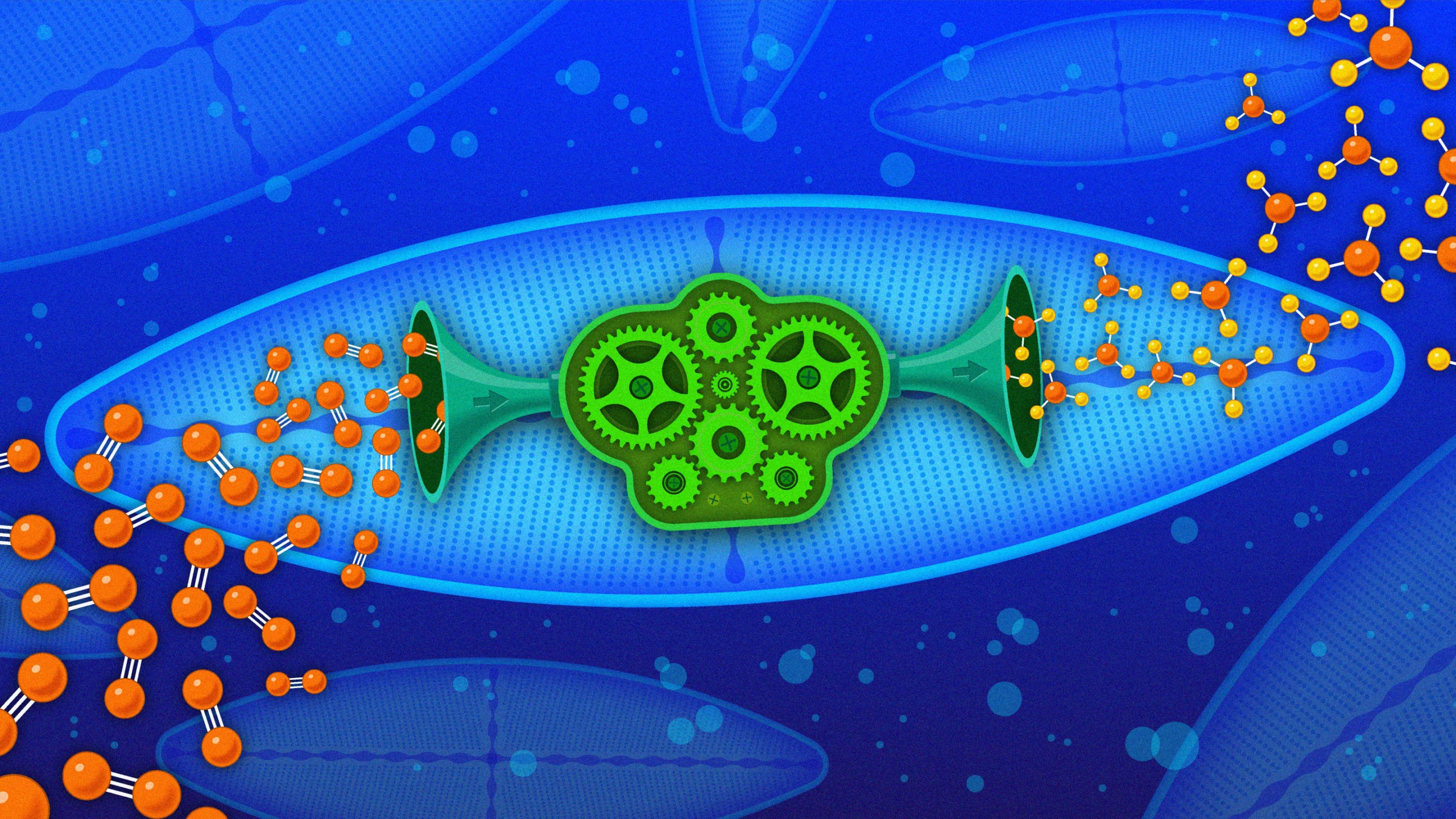
Across the world’s oceans, a pair of symbiotic microbes — bacteria that live inside the phytoplankton known as diatoms — do the essential work of making nitrogen available for life. Remarkably, the bacteria are related to those that “fix” most nitrogen on land.
Samuel Velasco/Quanta Magazine
Introduction
Nitrogen is fundamental to all life on Earth. Organisms use it to make amino acids and nucleic acids — the building blocks of proteins and DNA — among other vital molecules. Luckily, four-fifths of the atmosphere is nitrogen. Unluckily, in gaseous form it is inert and biologically unavailable: Every nitrogen atom is locked to another with a triple bond, which takes an extraordinary amount of energy to break. Without intervention, cells on land or sea cannot access this atmospheric source.
“We breathe it in, and we breathe it out, but we can’t do anything with it,” said Bernhard Tschitschko, a microbiologist at the Max Planck Institute for Marine Microbiology. “In life on Earth, nitrogen is one of the elements that controls growth.”
How, then, do organisms access this indispensable element? They rely on a select few bacteria with a special talent: the ability to convert nitrogen gas (N2) into ammonia (NH3), a process known as fixation, which makes the element available to life. All bacterial species that can break the triple bond of nitrogen gas do it using the same protein: nitrogenase. Every time a molecule of N2 is naturally converted into NH3, anywhere on Earth, it’s because of nitrogenase. The protein’s importance is reflected in how ancient it is: Nitrogenase emerged about 3.2 billion years ago in what researchers have called “one of the most consequential biogeochemical innovations over life’s history.”
For many decades, scientists were aware of only one genus, the filament-shaped cyanobacteria Trichodesmium, that seemed to be responsible for essentially all nitrogen fixed in the ocean. Then, in the 1990s and 2000s, new and inexpensive technologies let researchers scoop up a bucket of seawater and sequence the DNA of all critters living there. To their surprise, the scientists began discovering nitrogenase genes in the ocean that could not have come from Trichodesmium or any other cyanobacterium. Another organism, or more likely many, many organisms, were fixing nitrogen too.
“There are probably hundreds of different non-cyanobacterial nitrogenase genes in the ocean metagenome,” said Jonathan Zehr, an environmental microbiologist at the University of California, Santa Cruz. But researchers couldn’t pin down the organisms that harbored these alien nitrogenases. They shrugged and gave the non-Trichodesmium bacteria the umbrella name “Gamma A” until future researchers could identify them.

The microbiologist Bernhard Tschitschko was part of a team of researchers searching for the ocean’s missing nitrogen producers. They discovered a pair of microbes that work together to create the vital nutrient.
Max Planck Institute for Marine Microbiology
“We didn’t know anything about these organisms, who they are or whether they even fix nitrogen,” Tschitschko said. “All that was known about them was the nitrogenase sequence — nothing else.”
In a recent paper in Nature, Tschitschko and colleagues reported their discovery of two of these Gamma A organisms — closely related bacteria that live throughout the world’s oceans and supply the food web with nitrogen where Trichodesmium doesn’t. The bacteria don’t work alone: They are lodged firmly inside diatoms, an abundant microscopic phytoplankton, with which they trade nitrogen for housing and energy. The symbiotic relationship — a mutually beneficial collaboration between two independent organisms — is so tight that the bacterium may be on its way to becoming a permanent part of the diatom’s body as a new cellular organelle, according to a DNA analysis.
The partners’ lives at sea may feel distant from ours, but we have something in common. Most nitrogen on land is fixed by rhizobia bacteria, which live symbiotically in nodules on the roots of legume plants. The newly identified Gamma A organisms are related to rhizobia bacteria, suggesting an ancient genetic relationship between the two symbiotic partnerships that enable life on land and at sea.
Marine Detectives
Tschitschko had been working in his new lab at the Max Planck Institute for Marine Microbiology for only a few months when he set off on an expedition to discover Gamma A. He knew it wouldn’t be easy. In any given bucket of water, there might be millions or billions of microbial cells, and only a hundred might fix nitrogen.
Fishing with a bucket was almost exactly what Tschitschko and his team did: They dropped a CTD rosette — an array of large bottles, open but sealable at both ends — into the ocean to capture water at various depths. Later, in tanks hanging over the side of their ships, they mixed each sample with a form of nitrogen slightly different than the form produced in nature. Any cells that incorporated this alternate form of nitrogen (known as an isotope) within 24 hours must be able to fix nitrogen and incorporate it into their own proteins and DNA.

The researchers sampled microbial life in the western tropical North Atlantic using a CTD rosette — essentially a high-tech bucket — which samples water while taking measurements at various ocean depths.
Max Planck Institute for Marine Microbioloby
In the radioactive bacteria, Tschitschko and his colleagues detected the Gamma A version of the nitrogenase gene. They were on its trail. However, the gene was located in an exotic genomic environment. When they sequenced the DNA of the Gamma A bacterium, most of its genome was typical of a globally distributed class of bacteria called Alphaproteobacteria. Its nitrogenase gene, however, was taxonomically related to the land-based rhizobia.
If Gamma A’s genome is a chess set, its nitrogenase gene is a checkers piece thrown in the box: It has to have come from somewhere else.
This was odd enough, but the researchers still had not laid eyes on the organism in question, only its genome. Using genetic techniques, they tracked the rhizobia DNA to a marine diatom — one of the ubiquitous, photosynthetic microscopic algae of the sea — of the genus Haslea. Inside each diatom were four to eight bacterial cells. The cells turned out to be two bacterial species, which the researchers named Tectiglobus diatomicola and Tectiglobus profundi.
Haslea diatoms photosynthesize to create energy; then they hand over some of this energy to Tectiglobus, which supplies the diatom with nitrogen.
This mirrors the relationship between rhizobia and legumes on land, in which bacteria offer nitrogen to the plant in exchange for carbohydrates. Somehow, this nitrogenase gene found its way into two bacterial groups — and both went on to form symbiotic relationships, with very different host organisms, crucial for providing nitrogen to food webs.
To unpack these twisted histories, the researchers reconstructed evolutionary trees for the rhizobia and Tectiglobus bacteria. The results suggested that both groups acquired the ancient nitrogenase gene from other bacteria through horizontal gene transfer at different points in their evolutionary histories. The authors also speculated that Tectiglobus evolved its symbiotic relationship independently and earlier than its more widely known cousin onshore.
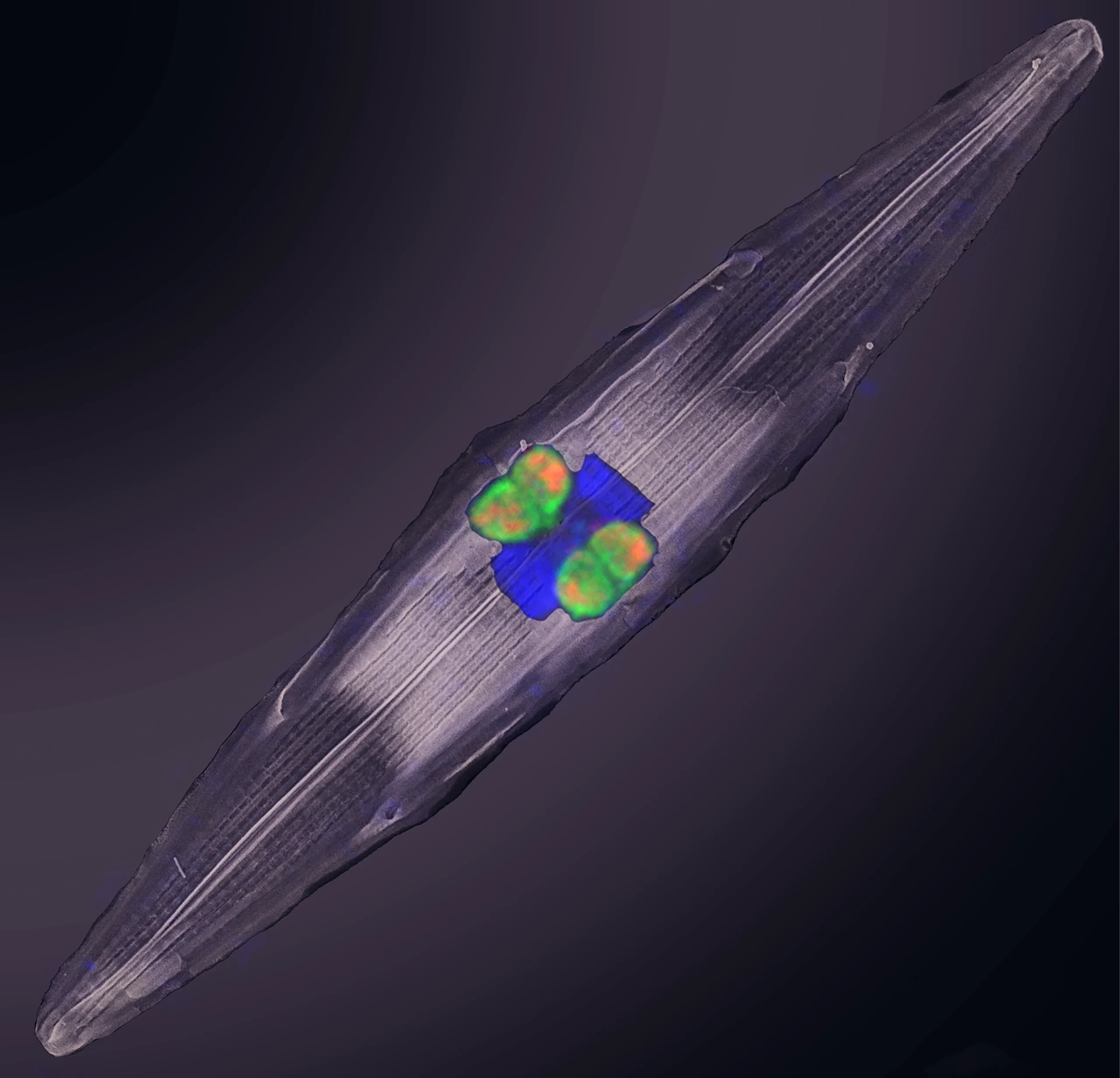
The symbionts in all their glory: Four cells of Tectiglobus bacteria, labeled in green, reside inside a glassy diatom (whose nucleus is shown in bright blue). The partners collaborate to produce nitrogen, which travels through the food web.
Mertcan Esti/Max Planck Institute for Marine Microbiology
Tectiglobus is doing important biochemical work in the ocean. The researchers estimated that Tectiglobus is fixing nitrogen at slightly less than half the rate of Trichodesmium, the cyanobacteria previously thought to dominate oceanic nitrogen fixation. And the Tectiglobus-diatom partners are found in oceans throughout the world. The relationship appears to represent a significant chunk of nitrogen fixation on Earth.
The Symbiotic Spectrum
It makes sense that a diatom would want to carry an in-house nitrogen source: The ocean is a desert. Nutrients are scarce, and most microbes are in a perpetual state of near-starvation. A photosynthesizing diatom with its own unlimited source of energy, but with a need for nitrogen, offered Tectiglobus a safe and beneficial arrangement.
“This is the way this one isolated, lonely little diatom can meet its own needs,” said Angelicque White, an oceanographer at the University of Hawaiʻi who wasn’t involved in the work. “These unusual associations break down our simplified description of how ecosystems work. They’re far from land. They’re far from the sources of nutrients. And so these organisms have to adapt in some way.”
But what is the arrangement exactly? It has a whiff of an enduring symbiotic relationship, but it’s also possible that Tschitschko caught the bacteria in the middle of a transition to full-fledged organelle, in which case they would cease to be an independent organism.
This is the same scenario that produced mitochondria and chloroplasts: Both organelles were formerly free-living bacteria that became symbionts of larger cells and eventually moved in permanently. The two Tectiglobus species, like mitochondria and chloroplasts, have a rather small genome, suggesting that they have been jettisoning genes they no longer need because the diatom host provides for them. When Tschitschko observed the host and symbiont dividing to reproduce, their divisions occurred together.
Both of these qualities — a diminished genome and paired reproduction — point to a long-lasting and stable symbiosis. Whether Tectiglobus is definitely on its way to losing even more of its genome and becoming an organelle requires more research.
“Undoubtedly there’s a spectrum of symbioses, from loose symbioses to an organelle, and these organisms can be placed along that spectrum,” said Zehr, who was not involved in the new research. In 2024, his lab reported a nitrogen-providing cyanobacterium that had become an organelle within an algal cell. Clearly, this is a recurring theme in the world of nitrogen fixation. After all, if you had the chance to manufacture your own vital nutrient by taking on a pet, how could you resist?
Correction: August 26, 2024
A sentence was revised to correct how the researchers traced nitrogen in the experiment: using isotopes not radioactivity.
Correction: September 11, 2024
A sentence was revised to correctly describe the relationship between Gamma A organisms and rhizobia.