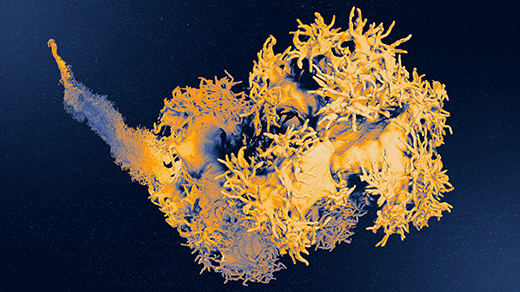
How Many Genes Do Cells Need? Maybe Almost All of Them
The activities of genes in complex organisms, including humans, may be deeply interrelated.
Olena Shmahalo/Quanta Magazine; Model by: TheEmptyRoom
Introduction
By knocking out genes three at a time, scientists have painstakingly deduced the web of genetic interactions that keeps a cell alive. Researchers long ago identified essential genes that yeast cells can’t live without, but new work, which appears today in Science, shows that looking only at those gives a skewed picture of what makes cells tick: Many genes that are inessential on their own become crucial as others disappear. The result implies that the true minimum number of genes that yeast — and perhaps, by extension, other complex organisms — need to survive and thrive may be surprisingly large.
About 20 years ago, Charles Boone and Brenda Andrews decided to do something slightly nuts. The yeast biologists, both professors at the University of Toronto, set out to systematically destroy or impair the genes in yeast, two by two, to get a sense of how the genes functionally connected to one another. Only about 1,000 of the 6,000 genes in the yeast genome, or roughly 17 percent, are considered essential for life: If a single one of them is missing, the organism dies. But it seemed that many other genes whose individual absence was not enough to spell the end might, if destroyed in tandem, sicken or kill the yeast. Those genes were likely to do the same kind of job in the cell, the biologists reasoned, or to be involved in the same process; losing both meant the yeast could no longer compensate.
Boone and Andrews realized they could use this idea to figure out what various genes were doing. They and their collaborators went about it deliberately, by first generating more than 20 million strains of yeast that were each missing two genes — almost all of the unique combinations of knockouts among those 6,000 genes. The researchers then scored how healthy each of the double mutant strains was and investigated how the missing genes could be related. The results let the researchers sketch a map of the shadowy web of interactions that underlie life. Two years ago, they reported the details of the map and revealed that it had already allowed researchers to discover previously unknown roles for genes.
Along the way, however, they realized that a surprising number of genes in the experiment didn’t have any obvious interactions with others. “Maybe, in some cases, deleting two genes isn’t enough,” Andrews said, reflecting on their thoughts at the time. Elena Kuzmin, a graduate student in the lab who is now a postdoc at McGill University, decided to go one step further by knocking out a third gene.
In the paper out today in Science, Kuzmin, Boone, Andrews and their collaborators at the University of Toronto, the University of Minnesota and elsewhere report that effort has yielded a deeper and more detailed map of the cell’s inner workings. Unlike in the double mutant experiments, the researchers did not make every possible combination of mutations — there are about 36 billion different ways to knock out three genes in yeast. Instead, they looked at the pairs of genes they’d already knocked out and ranked their interactions according to severity. They took a number of those pairs, whose effects ranged from making cells grow a little slower to making them significantly impaired, and matched them up one by one with knockouts of other genes, generating about 200,000 triple mutant strains. They monitored how quickly colonies of the mutant yeast grew, and after noting which mutants were struggling, they checked databases to see what the disabled genes were thought to do.
As the scientists built their new map, several things became clear. For one, in about two-thirds of the triple mutants that showed an additional genetic interaction, knocking out the third gene tended to intensify the problems that the double mutant had. Pairs of genes might already show some interaction with each other, Andrews said, “but it was much more severe when we deleted a third gene.” Boone says that these are likely to be situations in which the loss of a third gene is dealing a critical blow to an already faltering system.
However, a third of the interactions were completely new. And they tended to involve more disparate processes. In double mutants, the functional connections between genes tended to be tight: A gene involved in DNA repair usually had links with other genes that are also involved in DNA repair, and genes that had interactions with each other usually interacted with the same other genes. With the triple mutants, however, more far-flung tasks started to get linked together. The constellation of connected cellular tasks shifted and morphed subtly.
“Perhaps what we’re sampling here,” Andrews said, “are some functional connections in the cell that we weren’t able to see before.”
One set of new connections, for example, was between genes involved in transporting proteins and genes involved in DNA repair. On the surface, it’s difficult to see what would connect these two functions. And in fact, the researchers still don’t have a mechanistic explanation. But they are sure there is one. “Our immediate reaction was, ‘Well, that’s kind of random,’” Andrews said. “But we’ve learned over the course of doing this project that it’s not random. We just don’t understand how the cell is connected.”
Their group has just started probing that link between protein transport and DNA repair, but according to Andrews, if you look closely at those yeast cells, they do in fact show a great deal of DNA damage. The map of connections helped draw their attention to it: “There would have been no reason to look before,” she said.
Yeast geneticists were never under the impression that only essential genes mattered. But the new paper reinforces the idea that simplistic interpretations of just what is important in the yeast genome are likely to be flawed. The reality is more complicated, Boone and Andrews say. They suggest that when double and triple interactions are taken into account, the number of genes that a yeast cell truly can’t do without jumps. As their paper notes, the minimum genome needed for yeast cells to avoid a substantial defect “may nearly approach the complete set of genes encoded in the genome.”
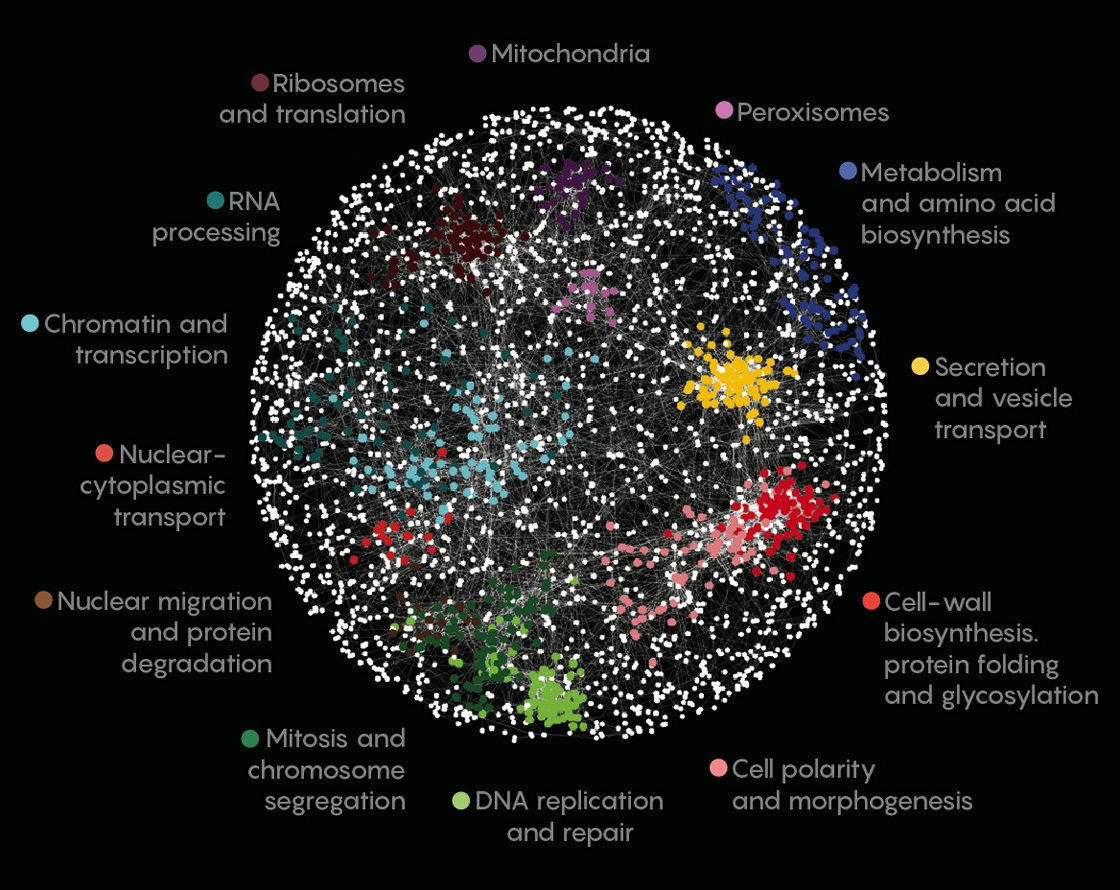
This figure maps the interactions among various genes (represented as dots) in the yeast genome. Genes with linked effects are connected by lines; genes with more strongly correlated effects are closer together. The color of the dots corresponds to the biological processes and organelles in which the genes are involved.
Anastasia Baryshnikova, University of Toronto
Indeed, experimental efforts to devise a minimal genome for a microorganism — to pinpoint the smallest number of genes that a cell would need to survive, as a step toward making artificial genomes — have shown it to be surprisingly difficult to remove genes and still have a thriving creature.
In 2016, researchers at the J. Craig Venter Institute (JCVI) reported the creation of an artificial genome for the bacterium Mycoplasma genitalium, in which they winnowed its 525 genes down to 473. But negative effects from removing seemingly inessential genes were indeed a serious issue, according to Clyde A. Hutchison III, a biochemist and distinguished professor at JCVI involved in the work. “That was the main problem for choosing a gene set to design for a minimal genome,” he said.
Joel Bader, a systems biologist at Johns Hopkins University, says that the current work suggests an intriguing connection to an idea in human genetics — that a wide array of genes may be subtly influencing traits that we don’t normally associate with them. “[The] closer we are able to look, the more we are able to see that perturbing one gene or pathway has effects that propagate throughout the entire system,” he said. “The effects get weaker, but they can still be measured.”
Ignorant as science may still be about certain happenings in yeast, it’s dwarfed by our ignorance of what is going on in our own cells. Part of what makes a project like this one at the University of Toronto possible is that yeast has been heavily studied and its genes intricately annotated by several generations of biologists, to a degree not yet reached with the human genome, which is comparatively enormous, rambling and full of mysteries. Still, the researchers say that they hope that as gene-editing technology for human cells advances, these kinds of experiments can help reveal more about the workings of cells and how the genes within a genome relate to one another. “I think there are many basic rules of genome biology we have not discovered,” Andrews said.
Correction: This article was updated on April 20 to include mention of the contributions of scientists at the University of Minnesota to the new Science paper. The credit for the map of gene interactions was also corrected on April 23 to read “Anastasia Baryshnikova, University of Toronto.”