Theorists Debate How ‘Neutral’ Evolution Really Is

The neutral theory of molecular evolution proposed by Motoo Kimura has dominated the landscape of evolutionary theory for half a century, but it continues to face challenges.
Maria Nguyen for Quanta Magazine
Introduction
When Charles Darwin articulated his theory of evolution by natural selection in On the Origin of Species in 1859, he focused on adaptations — the changes that enable organisms to survive in new or changing environments. Selection for favorable adaptations, he suggested, allowed ancient ancestral forms to gradually diversify into countless species.
That concept was so powerful that we might assume evolution is all about adaptation. So it can be surprising to learn that for half a century, a prevailing view in scholarly circles has been that it’s not.
Selection isn’t in doubt, but many scientists have argued that most evolutionary changes appear at the level of the genome and are essentially random and neutral. Adaptive changes groomed by natural selection might indeed sculpt a fin into a primitive foot, they said, but those changes make only a small contribution to the evolutionary process, in which the composition of DNA varies most often without any real consequences.
But now some scientists are pushing back against this idea, known as neutral theory, saying that genomes show much more evidence of evolved adaptation than the theory would dictate. This debate is important because it affects our understanding of the mechanisms that generate biodiversity, our inferences about how the sizes of natural populations have changed over time and our ability to reconstruct the evolutionary history of species (including our own). What lies in the future might be a new era that draws from the best of neutral theory while also recognizing the real, empirically supported influence of selection.
An “Appreciable Fraction” of Variation
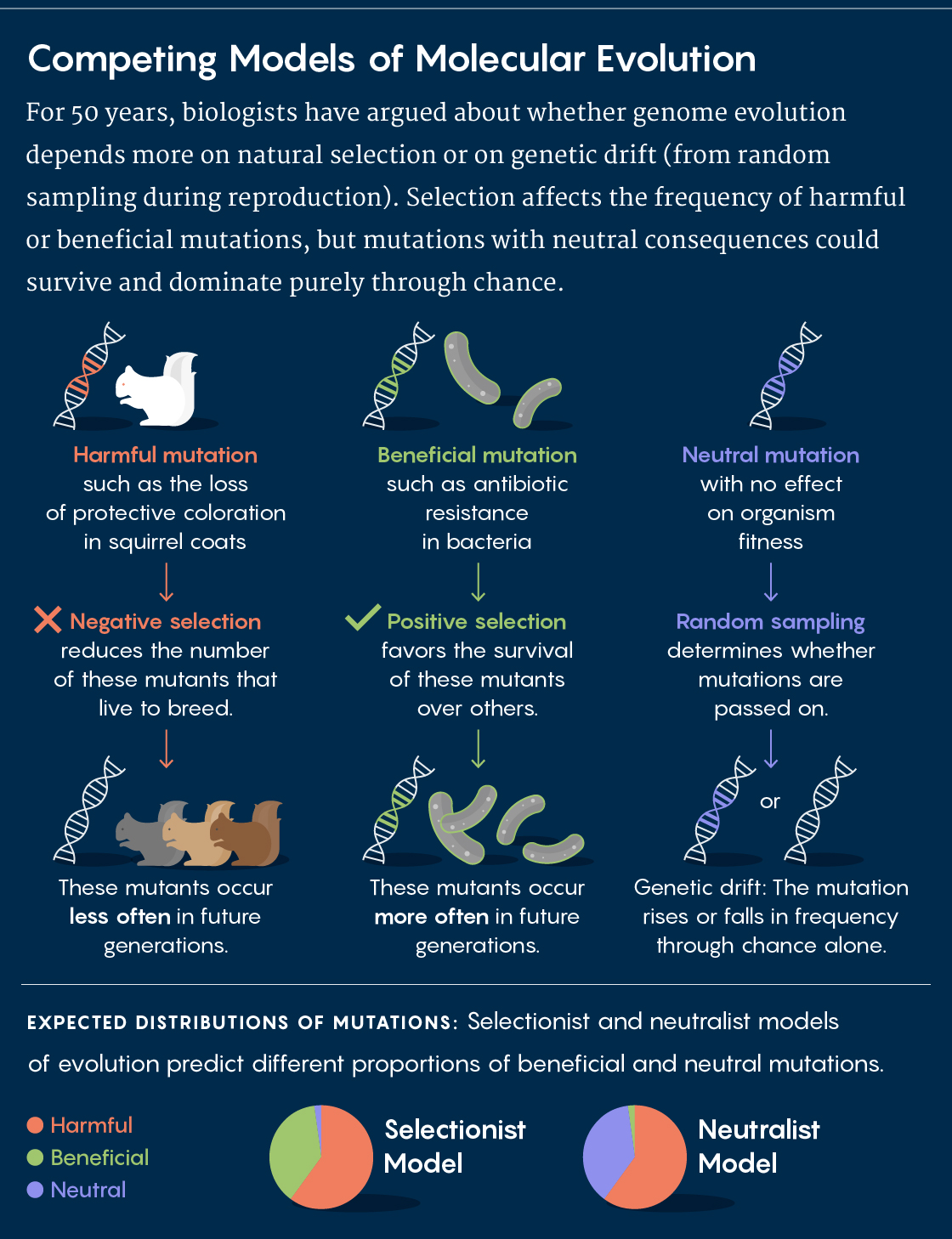
Darwin’s core insight was that organisms with disadvantageous traits would slowly be weeded out through negative (or purifying) selection, while those with advantageous features would reproduce more often and pass those features on to the next generation (positive selection). Selection would help to spread and refine those valuable traits. For most of the first half of the 20th century, population geneticists largely attributed genetic differences between populations and species to adaptation through positive selection.
But in 1968, the famed population geneticist Motoo Kimura resisted the adaptationist perspective with his neutral theory of molecular evolution. In a nutshell, he argued that an “appreciable fraction” of the genetic variation within and between species is the result of genetic drift — that is, the effects of randomness in a finite population — rather than natural selection, and that most of these differences have no functional consequences for survival and reproduction.
The following year, the biologists Jack Lester King and Thomas Jukes published “Non-Darwinian Evolution,” an article that likewise emphasized the importance of random genetic changes in the course of evolution. A polarized debate subsequently emerged between the new neutralists and the more traditional adaptationists. Although everyone agreed that purifying selection would weed out deleterious mutations, the neutralists were convinced that genetic drift accounts for most differences between populations or species, whereas the adaptationists credited them to positive selection for adaptive traits.
Much of the debate has hinged on exactly what Kimura meant by “appreciable fraction” of genetic variation, according to Jeffrey Townsend, a biostatistician and professor of evolutionary biology at the Yale School of Public Health. “Is that 50 percent? Is it 5 percent, 0.5 percent? I don’t know,” he said. Because Kimura’s original statement of the theory was qualitative rather than quantitative, “his theory could not be invalidated by later data.”
Nevertheless, neutral theory was rapidly adopted by many biologists. This was partly a result of Kimura’s reputation as one of the most prominent theoretical population geneticists of the time, but it also helped that the mathematics of the theory was relatively simple and intuitive. “One of the reasons for the popularity of the neutral theory was that it made things a lot easier,” said Andrew Kern, a population geneticist now at the University of Oregon, who contributed an article with Matthew Hahn, a population geneticist at Indiana University, to a special issue of Molecular Biology and Evolution celebrating the 50th anniversary of neutral theory.
To apply a neutral model of evolution to a population, Hahn explained, you don’t have to know how strong selection is, how large the population is, whether mutations are dominant or recessive, or whether mutations interact with other mutations. In neutral theory, “all of those very hard parameters to estimate go away.”
Lucy Reading-Ikkanda/Quanta Magazine
The only key input required by the neutral model is the product of the population size and the mutation rate per generation. From this information, the neutral model can predict how the frequency of mutations in the population will change over time. Because of its simplicity, many researchers adopted the neutral model as a convenient “null model,” or default explanation for the patterns of genetic variation they observed.
Some population geneticists were not convinced by Kimura’s argument, however. For instance, John Gillespie, a theoretical population geneticist at the University of California, Davis (and Kern’s doctoral adviser), showed in the early 1970s that some natural selection-based models could explain patterns observed in nature as well as neutral models, if not better.
More fundamentally, even when there aren’t enough data to disprove a neutral-theory null model, it doesn’t mean that natural selection isn’t happening, said Rebekah Rogers, an evolutionary geneticist at the University of North Carolina, Charlotte. “Any time you have limited data, the arguments get really fierce,” she said.
For decades, that was the crux of the problem: Kimura had proposed neutral theory at a time before inexpensive sequencing technology and the polymerase chain reaction became available, when gene sequence data were sparse. There was no simple way to broadly prove or disprove its tenets except on theoretical grounds because we didn’t know enough about genomic variation to resolve the dispute.
Strong Feelings About Neutrality
Today, 50 years after Kimura’s article, more affordable genomic sequencing and sophisticated statistical methods are allowing evolutionary theorists to make headway on quantifying the contribution of adaptive variation and neutral evolution to species differences. In species like humans and fruit flies, the data have revealed extensive selection and adaptation, which has led to strong pushback against Kimura’s original idea, at least by some researchers.
“The ubiquity of adaptive variation both within and between species means that a more comprehensive theory of molecular evolution must be sought,” Kern and Hahn wrote in their recent article.
Although the vast majority of researchers agree that strict neutrality as originally formulated is false, many also point out that refinements of neutral theory have addressed weaknesses in it. One of the original shortcomings was that neutral theory could not explain the varying patterns of genome evolution observed among species with different population sizes. For instance, species with smaller population sizes have on average more mutations that are deleterious.
To address this, one of Kimura’s students, Tomoko Ohta, now professor emeritus at Japan’s National Institute of Genetics, proposed the nearly neutral theory of molecular evolution in 1973. This modified version of neutral theory suggests that many mutations are not strictly neutral, but slightly deleterious. Ohta argued that if population sizes are large enough, purifying selection will purge them of even slightly deleterious mutations. In small populations, however, purifying selection is less effective and allows slightly deleterious mutations to behave neutrally.
Nearly neutral theory also had problems, Kern said: It did not explain, for example, why the rate of evolution varies as observed among different lineages of organisms. In response to such challenges, Ohta and Hidenori Tachida, now a professor of biology at Kyushu University, developed yet another variation of the nearly neutral model in 1990.
Opinions about the standing of nearly neutral theory can still differ sharply. “The predictions of nearly neutral theory have been confirmed very well,” said Jianzhi Zhang, who studies the evolution of genomes at the University of Michigan and also contributed to the special issue of Molecular Biology and Evolution.
Kern and Hahn disagree: Nearly neutral theory “didn’t explain much from the start and then was shuffled around in attempts to save an appealing idea from the harsh glare of data,” Kern wrote in an email.
How Much Evolves Neutrally?
For Townsend, the ongoing debate between neutralists and selectionists isn’t particularly fruitful. Instead, he said, “it’s just a quantitative question of how much selection is going on. And that includes some sites that are completely neutral, and some sites that are moderately selected, and some sites that are really strongly selected. There’s a whole distribution there.”
When Townsend first started studying cancer about a decade ago after training as an evolutionary biologist, he saw that cancer biologists had begun to study mutations at a level of detail that could reveal information about mutation rates at individual sites in the genome. That’s precious information that most population geneticists don’t get from the wild populations they study. Yet few cancer biologists study natural selection, and that’s what Townsend has brought to the cancer field with his background in evolutionary biology.
In a paper published in late October in the Journal of the National Cancer Institute, Townsend and his Yale colleagues presented the results of their evolutionary analysis of mutations in cancers. “What we’ve been able to do is actually quantify, on a site-by-site basis, what the selection intensities of different mutations are,” he said. Cancer cells are rife with mutations, but only a small subset of those are functionally important to the cancer. The selection intensities reveal how important the different mutations are for driving growth in an individual case of cancer — and therefore which ones would be most promising as therapeutic targets.
“This quantification of the selection intensity is absolutely essential, I think, to guiding how we treat cancer,” Townsend said. “My point is, doctors today are encountering the question: Which drug should I give to this patient? And they don’t have a quantification of how important the mutations that those drugs target actually are.” Someday, Townsend hopes, this evolutionary framework will offer a genetic basis for choosing the right drug and even predicting how a specific tumor might develop resistance to a treatment.
Although identifying the mutations undergoing the strongest selection is clearly useful and important, selection can also have subtle but important indirect effects on regions of the genome neighboring the target of selection.
The first hint of these indirect effects came in the 1980s and ’90s with the advent of the polymerase chain reaction, a technique that enabled researchers to look at nucleotide-level variation in gene sequences for the first time. One thing they discovered was an apparent correlation between the level of genetic variation and the rate of recombination at any specified region of the genome.
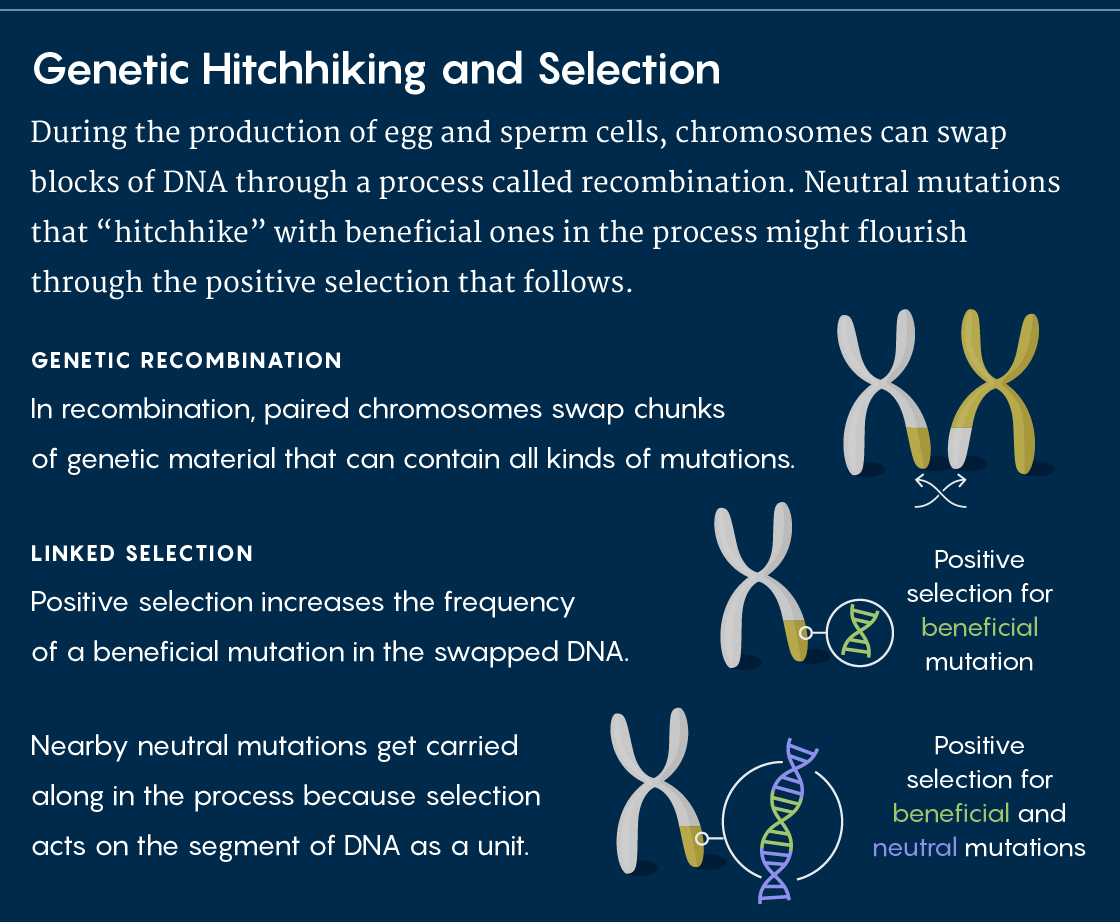
Recombination is a process in which the maternal and paternal copies of chromosomes exchange blocks of DNA with each other during meiosis, the production of sperm and egg cells. These recombinations shuffle genetic variation throughout the genome, splitting up alleles that might have previously been together.
By 2005, researchers could get whole-genome data from a variety of organisms, and they started to find this apparent correlation between levels of genetic variation and the rates of recombination everywhere, Kern said. That correlation meant that forces beyond direct purifying selection and neutral drift were creating differences in levels of variation across the genomic landscape.
Kern argues that the differences in the rates of recombination across the genome reveal a phenomenon called genetic hitchhiking. When beneficial alleles are closely linked to neighboring neutral mutations, natural selection tends to act on all of them as a unit.
Genetic hitchhiking meant that evolutionary geneticists suddenly had a whole new force called linked selection to worry about, Kern said. If only 10 percent of the genome is under direct selection in a population, then linked selection means that a much larger percentage — maybe 30 or 40 percent — might show its effects.
Lucy Reading-Ikkanda/Quanta Magazine
And if that is true, then selection for adaptive variants indirectly shapes neighboring genomic regions, leading to “a situation where neutral alleles have their frequencies determined by more than genetic drift, and instead have a new layer of stochasticity induced by selection,” Kern explained by email: Linked selection would produce more variance between generations than one would expect under neutrality.
Zhang points out that linked neutral mutations are still neutral. They might be hitchhiking with beneficial alleles, but that linkage is random — they could as easily be linked to harmful alleles and weeded out through “background selection.” So the neutral mutations’ fate is still determined by chance.
Kern agrees: The neutral mutations are still neutral — but they are not behaving as neutral theory would predict. Purifying selection at linked sites, he wrote, would “add noise to allele frequencies beyond drift,” while background selection and hitchhiking would lead to less genetic variation than under neutrality.
Neutral Models and Human Evolution
“While neutral models have without doubt begat tremendous theoretical fruits … the explanatory power of the neutral theory has never been exceptional,” Kern and Hahn wrote in their paper. “Five decades after its proposal, in the age of cheap genome sequencing and tremendous population genomic data sets, the explanatory power of the neutral theory looks even worse.”
In humans, recent evidence suggests “there’s a lot more adaptation than we ever thought was present,” Kern said. Recent human evolution is largely a history of migrations to new geographical locations where humans encountered new climates and pathogens to which they had to adapt. In 2017, Kern published a paper showing that most human adaptations arose from existing genetic variation within the genome, not novel mutations that spread rapidly through the population.
Even so, only about 1 percent of the human genome actually codes for proteins, said Omar Cornejo, an evolutionary genomicist at Washington State University. Maybe about 20 percent of the genome regulates when and where those coding regions are expressed. But that still leaves about 80 percent of the genome with unknown function.
Parts of this noncoding portion of the genome are riddled with repetitive DNA sequences, caused by transposable genetic elements, or transposons, that copy and insert themselves throughout the genome. According to Irina Arkhipova, a molecular evolutionary geneticist who studies the role of transposons at the Marine Biological Laboratory at the University of Chicago, “this portion of the genome is quintessentially neutral in Kimura’s sense,” even if some fraction of those transposons do affect the expression of genes. Because of this, neutral models applied to the nonfunctional regions of the genome can be used to infer the demographic history of human populations (and a variety of other organisms) quite accurately, Cornejo said.
Kern disagrees. “I’d argue we have no idea if we are accurately estimating human demographic history,” he wrote in an email. If you computationally simulate a population evolving neutrally, then methods for estimating demography will work; but introduce linked selection, and those methods fail.
Kern is agnostic about what percentage of the human genome is functional, but he believes that genetic linkage touches a large — yet unknown — fraction of the genome. With the accumulating evidence for adaptation in the human genome, it seems likely that some large fraction of the genome would be subject to the effects of linked selection, he suggested. “We just don’t know how large that fraction is.”
A recent paper in eLife by Fanny Pouyet and her computational-geneticist colleagues at the University of Bern and the Swiss Institute of Bioinformatics pins down that number. “Up to 80-85 percent of the human genome is probably affected by background selection,” the authors wrote.
After they additionally accounted for biased changes in genes that recombination can introduce during DNA repair, they concluded that less than 5 percent of the human genome evolved by chance alone. As the editors of eLife noted in their summary of the paper, “This suggests that while most of our genetic material is formed of non-functional sequences, the vast majority of it evolves indirectly under some type of selection.”
It’s possible that this estimate may creep even higher as biologists learn to recognize more subtle hints of selection. The new frontier in population genomics is focusing on traits such as height, skin color and blood pressure (among many others) that are polygenic, meaning that they result from hundreds or thousands of genes acting in concert. Selection for greater height, for instance, requires piling up changes at a number of dispersed genes to have an effect. Similarly, when farmers select strains of corn for higher yields, the impact generally shows up in many genes simultaneously.
But detecting polygenic adaptations in natural populations is a “very tricky business,” according to Kern, because those multitudes of genes are likely to be interacting in complex, nonlinear ways. The statistical methods for spotting those suites of changes are only beginning to be developed. To Kern, it will involve learning to appreciate “a whole other flavor” of adaptation because it will involve many small changes in individual mutation frequencies that collectively matter to natural selection.
In other words, it’s yet another non-neutral mechanism affecting genome evolution. As useful as the neutral theory has been in its various forms over the past half-century, the future of evolutionary theory may inevitably depend on finding ever-better ways to do the hard work of figuring out exactly how — and how much — selection is inexorably shaping our genomes after all.
Correction added on Nov. 9: The original version of this article misstated the key input required by the neutral model.