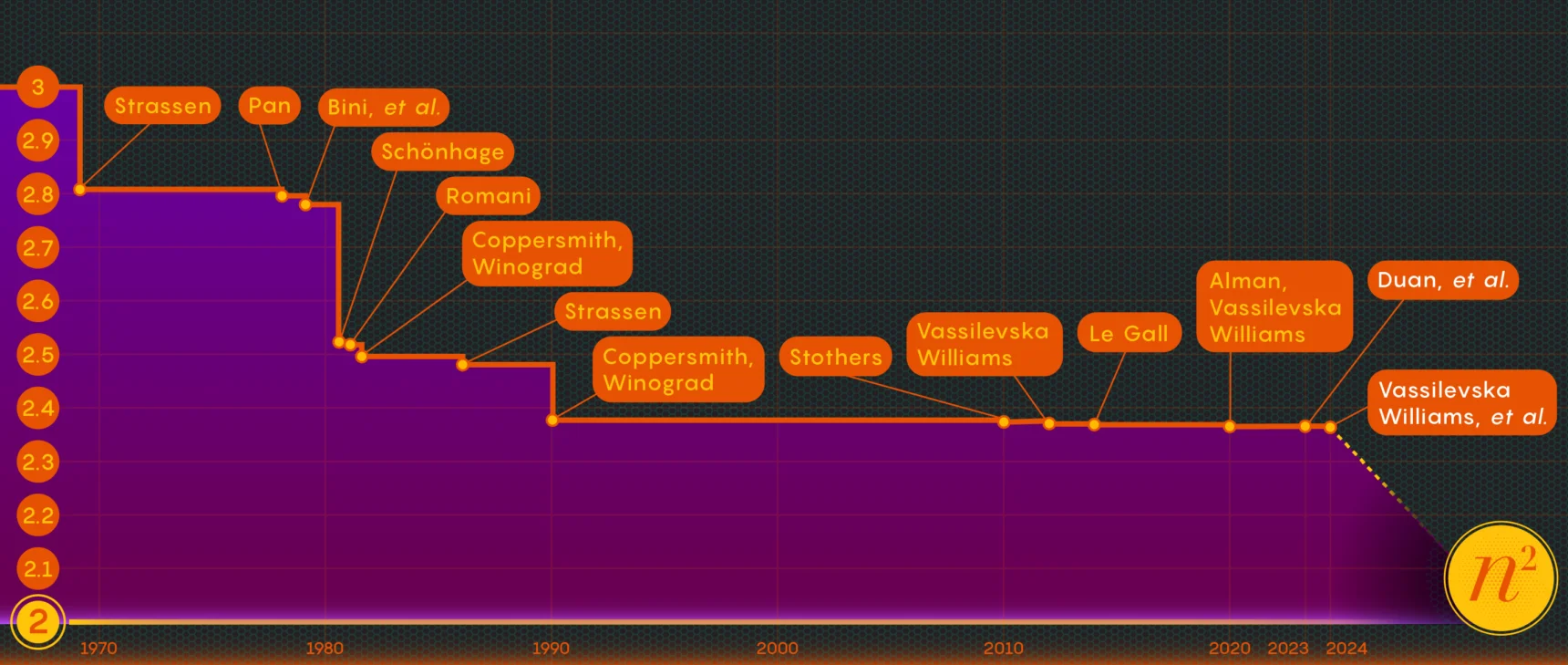
Tiny Tweaks to Neurons Can Rewire Animal Motion
Introduction
In March 2019, on a train headed southwest from Munich, the neuroscientist Maximilian Bothe adjusted his careful grip on the cooler in his lap. It didn’t contain his lunch. Inside was tissue from half a dozen rattlesnake spinal cords packed in ice — a special delivery for his new research adviser Boris Chagnaud, a behavioral neuroscientist based on the other side of the Alps. In his lab at the University of Graz in Austria, Chagnaud maintains a menagerie of aquatic animals that move in unusual ways — from piranhas and catfish that drum air bladders to produce sound to mudskippers that hop around on land on two fins. Chagnaud studies and compares these creatures’ neuronal circuits to understand how new ways of moving might evolve, and Bothe was bringing his rattlesnake spines to join the endeavor.
The ways that animals move are just about as myriad as the animal kingdom itself. They walk, run, swim, crawl, fly and slither — and within each of those categories lies a tremendous number of subtly different movement types. A seagull and a hummingbird both have wings, but otherwise their flight techniques and abilities are poles apart. Orcas and piranhas both have tails, but they accomplish very different types of swimming. Even a human walking or running is moving their body in fundamentally different ways.
The tempo and type of movements a given animal can perform are set by biological hardware: nerves, muscle and bone whose functions are bound by neurological constraints. For example, vertebrates’ walking tempos are set by circuits in their spines that fire without any conscious input from the brain. The pace of that movement is dictated by the properties of the neuronal circuits that control them.
For an animal to evolve a novel way of moving, something in its neurological circuitry has to change. Chagnaud wants to describe exactly how that happens.
“In evolution, you don’t just invent the wheel. You take pieces that were already there, and you modify them,” he said. “How do you modify those components that are shared across many different species to make new behaviors?”
Recently, his team found one answer to this question in their experiments with Bothe’s rattlesnakes — an organism that has two distinct movement tempos built into one long, slender body.

A rattlesnake’s two distinct ways of moving — a slow slithering of its body and a rapid rattling of its tail — makes it an ideal experimental setup for studying how molecular changes in motor neurons can produce different movements.
T. Kohl
Their results, published in Current Biology in January, identified how tinkering with a single protein — a potassium ion channel — could make quick-firing motor neurons from a snake’s rattling tail behave more like the sluggish motor neurons from its undulating body, and vice versa. The finding is evidence that seemingly minute changes to an animal’s physiology can translate the same command from the nervous system into different ways of moving.
“What I thought was particularly unique and interesting about this study is that they focused on motor neurons with two very different jobs, but within the same animal,” said the neuroscientist Martha Bagnall of Washington University in St. Louis, who wasn’t involved in the work. “Looking at them within one animal gave them this really nice, tight comparison.”
The finding points to a way that animals across the tree of life can evolve new behaviors. Tweaking the right piece of biological machinery — in this case, a specific ion channel — can drastically change performance, just as twisting the volume dial does on a loudspeaker. Evolution can act first on the controls, rather than reworking the entire machine.
“It was a very clean result,” said Paul Katz, a behavioral neuroscientist at the University of Massachusetts, Amherst, who was also not involved in the work. “And, you know, rattlesnakes — they’re cool.”
Setting Screws
Chagnaud isn’t interested in rattlesnakes per se. “I just saw an interesting biological question,” he said. “I’m a science opportunist.”
His team studies organisms they think will reveal what they call behavior’s evolutionary Stellschrauben. The German word literally means “setting screws,” though that’s an awkward translation: Stellschrauben are the small controls that adjust the settings of a greater machine. If the machine is the nervous system and the settings are direct behavior, the Stellschrauben are the biological switches, triggers and knobs that, with just a little tweak, change an animal’s conduct dramatically enough to have evolutionary consequences.
Rattlesnakes present an opportunity to understand how biology changes its speed settings in a single animal. Researchers interested in such questions often have to compare different species with contrasting behaviors — say, a seagull and a hummingbird, both of which fly, but with different movements at different speeds. However, in that case it is hard to pin down which of the many biological distinctions between the two species underpins variation in a single movement behavior. Comparing a rattlesnake’s slow slithering to its rapid rattling avoids the problem of comparing apples to oranges, or anchovies to orcas.

Boris Chagnaud (left) and Maximilian Bothe are looking to identify evolution’s Stellschrauben, which is German for “setting screws” — the biological dials that can be twisted to generate new behaviors.
Uni Graz/Tzivanopoulos
That insight — that rattlesnakes have two ways of moving in one body — is why Bothe found himself sitting on a train from Munich to Graz with a cooler full of snake spines.
Back in Graz, he embedded the rattlesnake spinal tissue in agar, a type of gelatin, and made razor-thin slices for microscopy. Visually, motor neurons from the snake’s rattle and body seemed exactly the same. But when Bothe used an electrode to test their electrical properties, he found striking differences.
Neurons alter their electrical activity using pumps and channels embedded in their cell membranes to control the flow of charged ions like potassium and sodium. At rest, neurons keep their insides more negatively charged than their outside environment, maintaining a resting membrane voltage of about −70 millivolts. Then, when signals from other neurons increase this membrane voltage, the cell “fires” — it throws open the floodgates of its ion channels and allows positive ions to flow inside, producing a rapid voltage spike.
This voltage spike, called an action potential, zips along the neuron’s cell membrane until it reaches a synapse, the interface between a neuron and another cell, where it triggers the release of messenger chemicals called neurotransmitters. In the case of motor neurons and a muscle, the release of the neurotransmitter acetylcholine tells the muscle to contract.
Bothe found that the electric current needed to reach the voltage threshold and trigger a snake’s body motor neuron was “way lower than for the rattle motor neurons,” he said. “You need to put way more current into the [rattle] neuron for it to fire.” And compared to rattle motor neurons, body motor neurons reacted more sluggishly.
Merrill Sherman/Quanta Magazine
Merrill Sherman/Quanta Magazine
Because rattle neurons fire only in response to big, obvious signals, they are less likely to misfire because of weak fluctuations in the neurological background noise. They’re less jumpy and more precise, which allows them to relay higher-frequency signals.
Having identified this difference between rattle and body motor neurons, the next step was to find the Stellschrauben that control it.
Trial and Error
Neurons are cells, not machines, which means they have messy biological complexity. The “screw” Bothe and Chagnaud were looking for that controlled the motor neuron’s electrical properties could be anything from a subtle tweak in the structure of a membrane protein to the expression of an entirely different set of ion pumps and channels. Still, the researchers had good reason to think their Stellschrauben would involve a potassium ion channel. Previous studies of neurons had established that these channels are important for tuning neurons’ precision, but their role in adjusting the behavior of motor neurons specifically was unclear.
“There’s a certain toolkit, let’s say, that is available to evolution,” Bothe said. “So maybe it’s the same ion channels here.”
Finding the exact channel took years of trial and error. Comparing how body and rattle cells expressed genes for potassium channels didn’t reveal any significant differences. So Chagnaud and Bothe plowed ahead by testing the effects of drugs designed to block specific types of channels. Finally, they found a channel that, when blocked, generated different movement speeds: a potassium channel called KV72/3.
Bothe then performed more precise experiments, using drugs to enhance and hinder the channel’s activity. When he restricted the channel in rattle motor neurons, they fired more sluggishly and imprecisely, as if they were body motor neurons. Then, when he enhanced the potassium ion channel, he observed the opposite effect: Body motor neurons fired quickly and precisely, like rattle motor neurons.
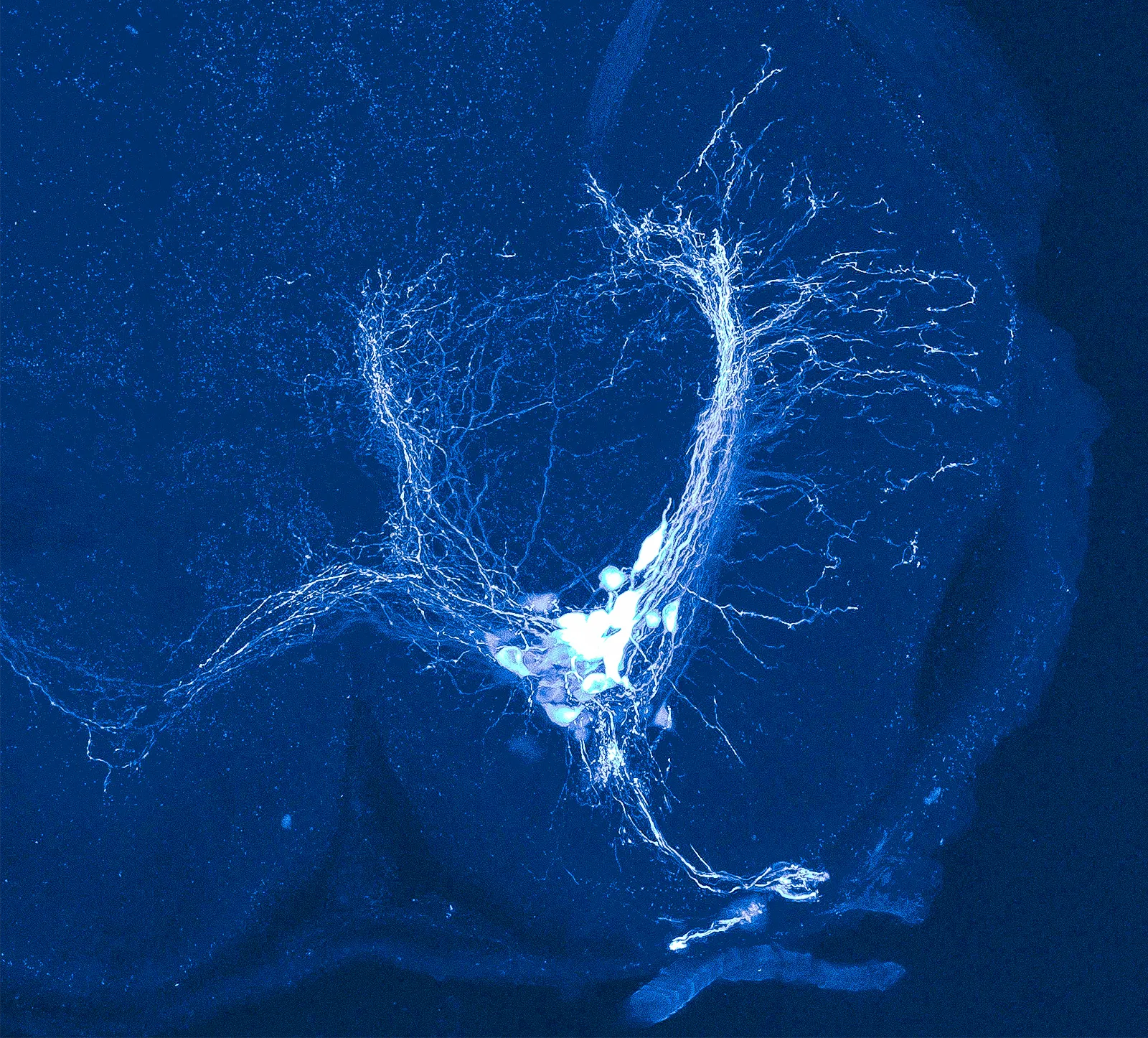
A rattlesnake’s motor neurons, which innervate the muscles of its rattle, glow bright in this spinal cord slice. Researchers discovered that alterations to an ion channel, which modulates a cell’s voltage, made a rattle neuron behave like a body neuron.
Max Bothe
It was as if this ion channel were a dial that could twist one neuron type into the other. But what was actually different about this protein in the snake’s body and rattle?
At first, the researchers thought that rattle motor neurons must have extra KV72/3 potassium channels. If the rattle neurons had more channels, the scientists figured, then they could discharge ions more quickly, bringing the voltage back down to prepare the channels to quickly fire again.
To find out, Bothe and Chagnaud extracted and sequenced RNA from both types of rattlesnake motor neurons and sent the data to Jason Gallant, an evolutionary biologist at Michigan State University, so he could compare the expression of the KV72/3 channel gene between the two tissues. The gene for KV72/3 channels is the same in every cell of the animal’s body — but if the rattle neurons had more KV72/3 channels, the researchers would expect to see higher gene expression in that tissue.
Alas, their simple explanation was not proved out. “There really is no difference in the level of gene expression in these potassium channels, which was disappointing,” Gallant said. “But I think it opens up a more realistic view of biology.”
Variations in the gene’s expression would have provided a simple, open-and-shut way to explain how the evolutionary screws on rattlesnake motor neurons are adjusted. But biology offers other possibilities. Chagnaud and Bothe speculated that after the channel proteins are constructed from the genetic blueprint, they could be modified into slightly different forms that manage ions differently. More research will be needed to pin down the details — to find the control that adjusts the control.
For his part, Katz didn’t consider the result disappointing at all. “So they didn’t see a [change in] gene expression. That was the answer they expected,” he said. “But the fact is that that’s a cool result.”
For many decades, researchers have assumed that motor circuits “exist as they will be used,” Katz said — meaning that initiating a behavior like walking or swimming is simply a matter of turning on the right circuit. In this view, evolving a new behavior would require an entirely new circuit layout. But in studies of organisms as diverse as crustaceans, sea slugs and now possibly snakes, researchers are finding that interactions with neuromodulators and other chemicals can modulate the activity that a circuit evokes, leading the same networks of cells to produce markedly different behaviors.
The new study, Katz said, hints that playing with this plasticity could be a way that new movement behaviors evolve. Perhaps the difference between rattle and body behavior has something to do with subtle differences in their cells’ chemical environments, not the structure or expression of the ion channel itself.
“For a lot of evolutionary modifications, your primary goal is to not break the animal, right?” Bagnall said. “Anything that you can do that tunes traits without becoming an on/off switch is a powerful means of driving change without being deeply deleterious.”
Turning and Tuning
This new study shows that it’s possible to tune motor neurons for wildly different behaviors by tweaking a single protein. But motor neurons are just one piece of the movement puzzle. They’re the last link in a chain that begins with circuits in the central nervous system known as central pattern generators, which generate the rhythmic patterns involved in walking or swimming. Those upstream circuits are better understood in other organisms, like zebra fish. In rattlesnakes, puzzling them out would be a next logical step.
“The number-one missing link,” Katz said, “is how do you create the frequency for the rattle? Where does that come from?”
Chagnaud is eager to find out if a similar Stellschraube tunes motor neurons in another species feared for its bite. Like rattlesnakes, piranhas execute two rhythmic movements with radically different frequencies: swimming, with a frequency of up to six cycles per second, and vibrating their swim bladders at frequencies of up to 140 cycles per second to make noises that sound like barks, yips and drumbeats. However, unlike rattlesnakes, piranhas use the same section of their spine to control both movement types.
“I’m curious to know, will it be KV72/3? We have no idea,” Chagnaud said. “Did evolution find the same solution to the same problem?”
He has his doubts. Although he’s hopeful about finding a similar mechanism, the surprising — and at times frustrating — discovery in rattlesnakes “was an eye-opener,” he said. Evolution is not a human designer with a goal in mind. Its methods are mysterious, and its toolbox is vast. “And you have very different screws that you can turn.”