To Defend the Genome, These Cells Destroy Their Own DNA

Allison Li for Quanta Magazine
Introduction
Marie Delattre was studying the sexual reproduction practices of microscopic worms when she noticed something unexpected. Under the microscope, an embryo of the nematode Mesorhabditis belari was dividing as it should, progressing from one cell to two to four. But inside a few cells she saw an inexplicable spray of DNA fragments floating around where they didn’t belong.
“There was DNA everywhere, inside the nuclei and outside the nuclei — big chunks of DNA,” she said. “I thought it was a dead embryo.”
The embryo was not dead, but it was doing something that usually only dead cells do: destroying its genome. “I started to try to trace when these fragments appear, at what stage, and what they look like,” said Delattre, a cell biologist at the École Normale Supérieure in Lyon. “That’s how I figured out that this is not accidental. All the embryos did this.”
What Delattre had stumbled across, and what she and her lab described in a paper published in August in Current Biology, was an instance of programmed DNA elimination (PDE), in which organisms seem to purposefully eliminate portions of their genome. It’s an odd phenomenon that flies in the face of the precept that a genome is a vital, sacrosanct resource to be passed on faithfully to the next generation.
So far, researchers have identified PDE in only about 100 species across all branches of life: Single-celled ciliates with multiple nuclei do PDE; so do tiny worms, as well as the meters-long intestinal parasites of horses, many insects, and even songbirds. But PDE can be so difficult to spot that no one knows how common it really is. “It’s not very well known even among biologists,” Delattre said.
In addition to confirming the existence of another case of PDE, Delattre’s new paper also hints at an explanation for it. PDE points to a long-running fight between cells and DNA sequences that are of no use to their owner, or maybe even weigh it down. Like gardeners, cells must protect their genomes to remain functional and productive. What should a cell do when the weeds come in? The new study suggests that some species, like M. belari, might just pull the weeds out using PDE.
Despite its seeming novelty, PDE was discovered in the early days of molecular biology, long before researchers even knew that DNA is life’s genetic material. In 1887, the German biologist Theodor Boveri was studying the development of Parascaris, a nematode that parasitizes horses, when he witnessed its large genome coalesce, fragment and then reassemble into smaller portions during mitosis. The missing pieces were seemingly trashed without ceremony.
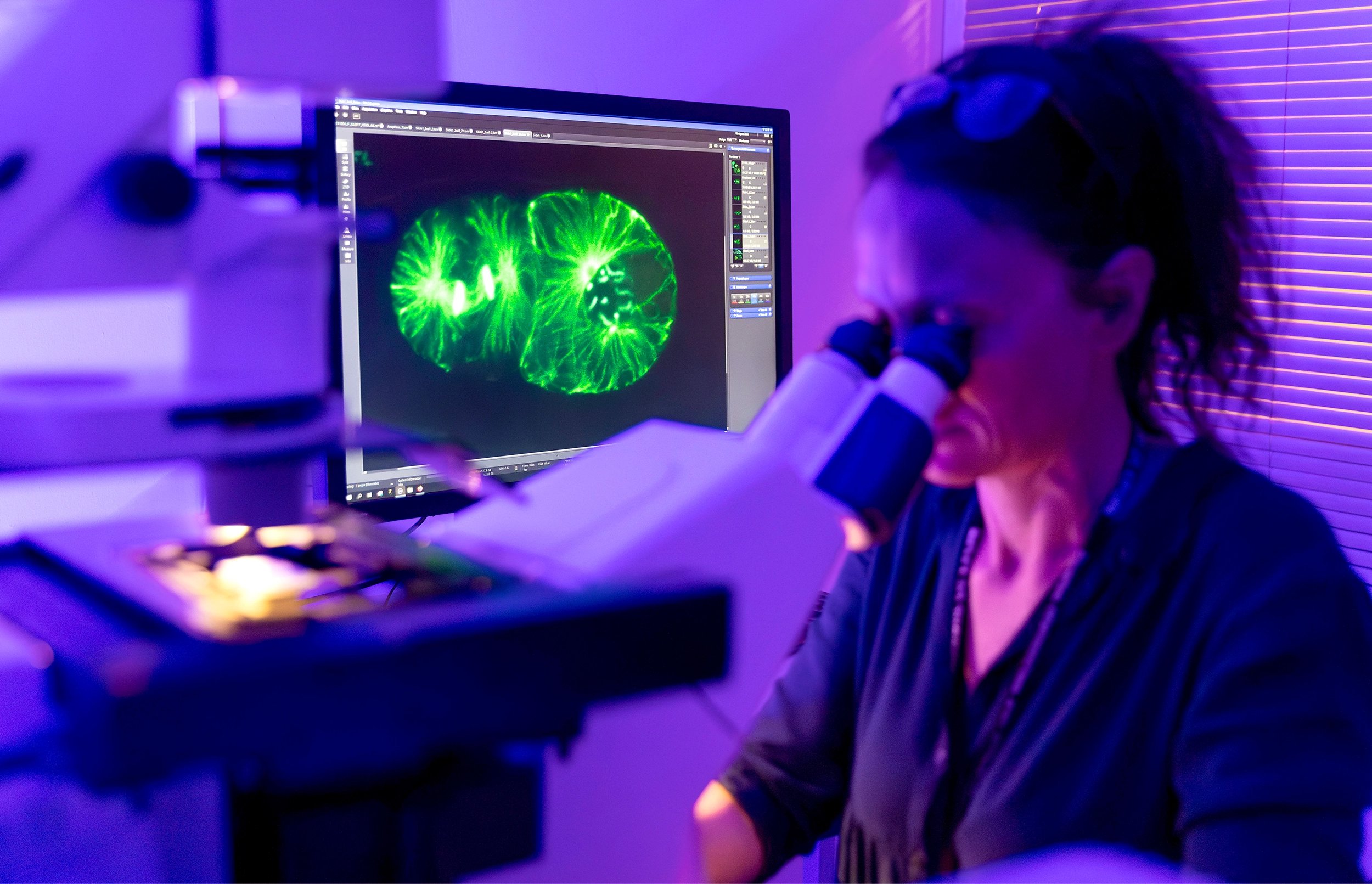
Marie Delattre, a cell biologist at the École Normale Supérieure in Lyon, was shocked to see “big chunks of DNA” floating in cells where they didn’t belong. The loose DNA encoded long strings of repeated sequences — potentially harmful genomic parasites.
CNRS Simon Bianchetti
During the 20th century, researchers discovered only a handful of other organisms — ciliates, moths, copepods, bandicoots — that did PDE, and it remained a fringe concept. But why any of those species did it remained unclear.
To figure out what was going on, Delattre’s lab looked at the DNA of an adult worm. The researchers compared the genomes of M. belari’s germline cells — the specialized reproductive cells like sperm and eggs — with the genomes of the worm’s somatic (nonreproductive) cells. The somatic genomes were missing long strings of sequences present in germline genomes. Sometime between the embryo’s growth from seven cells to 32, huge chunks of DNA had vanished.
The scientists then watched nematode embryos develop under a microscope. As the cells grew and replicated their genomes, they broke 20 chromosomes down into fragments and then reassembled them into 40 miniature chromosomes. Most of the fragments rejoined in this new, smaller genome — but a substantial fraction were left out.
In total, the nematode deleted a whopping one-third of its genome. The deleted sequences weren’t selected at random. There was a pattern: They were mostly highly repetitive stretches of DNA that didn’t code for genes at all, Delattre found.
Similar stretches of repeating or noncoding sequences pack the genomes of eukaryotic cells. Some perform necessary functions. Satellite DNA, for instance, forms structures such as heterochromatin and centromeres that package DNA, while other repetitive sections regulate gene expression. However, some repeated sequences do not contribute to their host’s survival — and may even interfere with it.
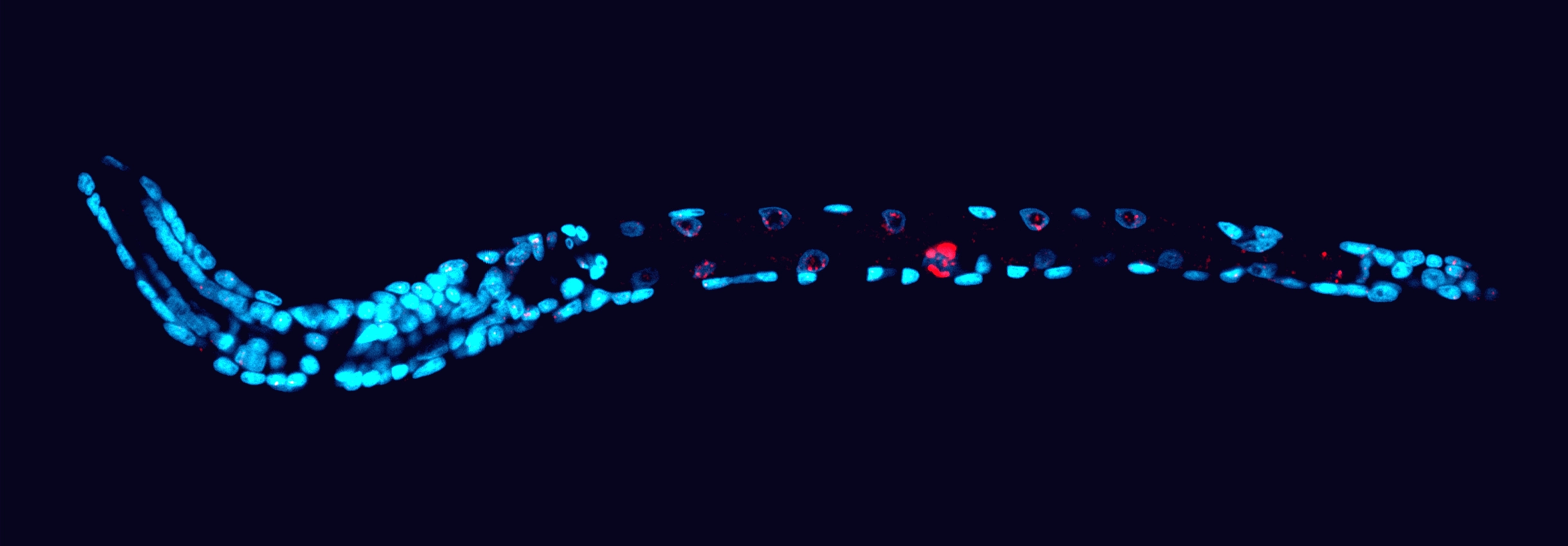
Scientists stained the DNA of this worm hatchling to hunt down the missing sequences. The deleted genome fragments (red) survive only in germline cells in the worm’s gonads; those fragments are absent from the nuclei of nearly all other body cells (blue).
Marie Delattre
That group includes transposons, self-replicating DNA sequences that steal the cell’s machinery to copy themselves by the thousands or millions. This amounts to molecular grand larceny, as well as a waste of the time and energy that the cell must spend to suppress these sequences. Cells routinely curb transposons with epigenetic marks that silence them, or by intercepting and destroying their RNA. But some species, such as M. belari, may remove them entirely through PDE.
That’s what Delattre suspects her nematodes are doing. Jonathan Wells, an evolutionary geneticist at Cornell University who studies transposons and was not involved in the new study, agrees that the DNA parasites are a likely target. For managing transposons, “the more you look, the more systems there are,” he said.
However, transposons and other types of self-replicating DNA are not necessarily villains. By copying themselves repeatedly across the genome, transposons also provide the cell with fresh material that can mutate and evolve into new genes. Host cells freely and liberally take genetic sequences from parasitic DNA and make them part of the normal genome — or, to look at it another way, the parasites ingratiate themselves with their hosts enough to be adopted. “[Repetitive DNA elements] are the soil that all other genes are sitting in,” Wells said. “They are a rich source of novelty.”
Since transposons can be both harmful and helpful, PDE might have uses aside from combating them. Even Delattre is not convinced that transposons are the whole story. Though the repetitive DNA that M. belari deleted was harmful, “why would you get rid of [parasitic DNA] just in somatic cells and not the germline?” she asked.
Besides fighting parasites, PDE may help cells streamline their genomes as they progress through different life stages. Many genes that are critical for an organism’s embryonic development are unnecessary in maturity. If a cell can rid itself of those genes, why wouldn’t it for efficiency’s sake? A larger genome is harder to copy and maintain, and inappropriately expressing developmental genes could cause problems. In somatic cells, which don’t need to pass a full genome along to offspring as germline cells do, removing unessential elements could be a winning evolutionary strategy.
No one knows for sure why PDE happens. Since it is under-studied and contradicts many deeply held genetic concepts (one paper, describing how some songbirds eliminate an entire chromosome, called these deletions “Mendelian nightmares”), almost any hypothesis could hold water.
That’s all the more reason for biologists to widen their search for it, Delattre said: “If it exists in other species we don’t know, we need to look for it.” By better understanding who is using PDE, scientists can get closer to understanding why some organisms would take such drastic and potentially risky measures to manage their genome. “I think it’s a good bet that PDE is more widespread than we know,” Wells said.